This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Patients with device-detected atrial fibrillation and multiple comorbidities do not benefit from anticoagulation: Study
In patients with device-detected atrial fibrillation and a high comorbidity burden, oral anticoagulation increases bleeding without a clear reduction in stroke. This is the main finding of a sub-analysis of the NOAH–AFNET 6 trial presented by Dr. Julius Nikorowitsch, University Medical Center Hamburg-Eppendorf (UKE), Hamburg, Germany, in a late-breaking science session at the annual congress of the European Heart Rhythm Association (EHRA) in Berlin, Germany, today (April 9) and simultaneously published in the European Heart Journal.
Device-detected atrial fibrillation (DDAF) are short and typically rare episodes of atrial fibrillation (AF) detected by pacemakers, defibrillators, and implanted loop recorders capable of continuous rhythm monitoring. Device-detected atrial fibrillation is found in every fifth patient with a cardiac implanted electronic device. Device-detected atrial fibrillation can lead to stroke, but the stroke risk in patients with device-detected atrial fibrillation appears lower than the stroke risk in patients with ECG-documented atrial fibrillation.
Recently, the NOAH–AFNET 6 (Non vitamin K antagonist Oral anticoagulants in patients with Atrial High-rate episodes) trial found that anticoagulation expectedly increases bleeding events in patients with device-detected atrial fibrillation while the stroke preventing effect was smaller than expected. This even applies to patients with long episodes of device-detected AF. A meta-analysis of NOAH–AFNET 6 confirmed an increase in bleeding and detected a small reduction in ischemic strokes with anticoagulation.
Dr. Nikorowitsch explained, "Older age, female sex, kidney disease, diabetes, heart failure, and other comorbidities increase the risk of adverse cardiovascular outcomes. We were interested if oral anticoagulation might reduce cardiovascular event rates in patients with a high burden of these factors and device-detected AF. To address this, we performed a prespecified secondary analysis of the NOAH–AFNET 6 data set."
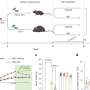
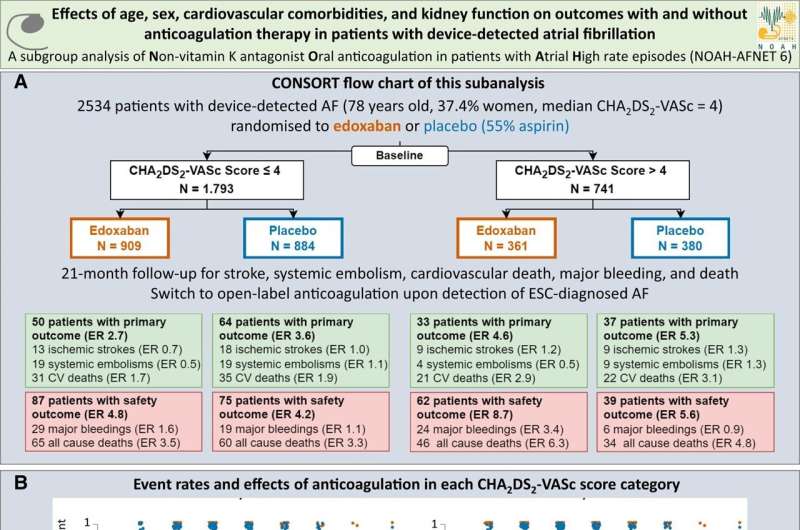
NOAH–AFNET 6 randomized 741 elderly patients (mean age 79 years, 52% women) with device-detected AF and multiple comorbidities (CHA2DS2-VASc >4) into two cohorts: one receiving anticoagulation with edoxaban, the other without anticoagulation. This patient population was outside of the approved indication of edoxaban. The stroke rate was low without anticoagulation (1.3%/year).
Edoxaban did not considerably reduce thrombo-embolic events, but increased bleeding and death in this patient group. Older age, diabetes, and kidney disease independently predicted the primary outcome defined as a composite of stroke, systemic embolism, or cardiovascular death. Anticoagulation, age, heart failure, diabetes, prior stroke, and kidney disease predicted safety outcome defined as major bleeding event or death.
Prof. Paulus Kirchhof, UKE, principal investigator of the NOAH–AFNET 6 trial, concluded: "The findings are consistent with the main trial: The stroke rate in patients with device-detected AF is low, even with multiple comorbidities. Anticoagulation had only a minor effect on stroke and systemic embolism.
"Our results, together with other data and considering the limitations of all subgroup analyses, can help to adapt the safe and effective use of oral anticoagulants in patients with device-detected AF and multiple stroke risk factors in clinical practice, and in future guidelines. They also call for new methods to identify patients with device-detected AF at high risk of stroke which might benefit from anticoagulation."
More information: Paulus Kirchhof et al, Oral anticoagulation in device-detected atrial fibrillation: effects of age, sex, cardiovascular comorbidities, and kidney function on outcomes in the NOAH-AFNET 6 trial, European Heart Journal (2024). DOI: 10.1093/eurheartj/ehae225. academic.oup.com/eurheartj/adv … 41700?searchresult=1