This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Platform tracks SARS-CoV-2 mutational impact on disease severity, identifies effective therapeutic inhibitors
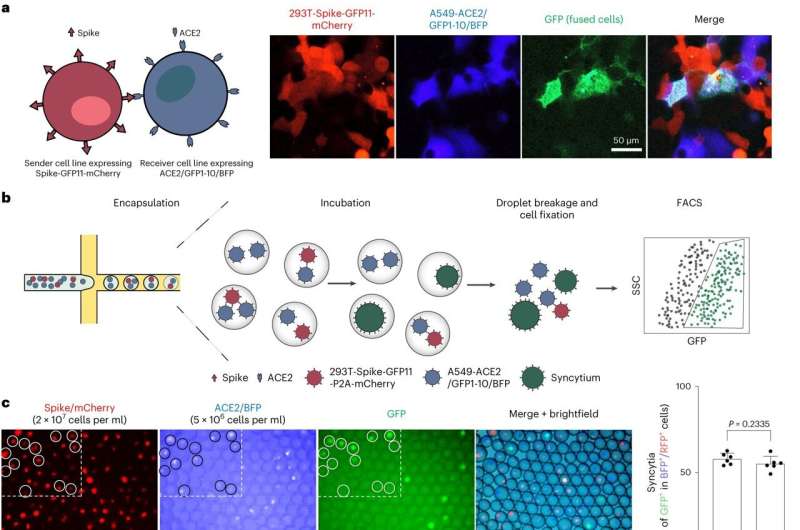
A multidisciplinary research team from the LKS Faculty of Medicine (HKUMed) and the Faculty of Engineering of the University of Hong Kong (HKU) has successfully developed an innovative screening platform to rapidly assess the impact of SARS-CoV-2 mutations on disease severity.
Compared to traditional methods, this platform has achieved a speed improvement up to 39 times faster. In addition, the researchers focused on understanding how mutations affect syncytium formation, a process known as cell fusion in which infected cells fuse with uninfected cells. This research helps identify emerging viral variants that could pose a significant risk to public health.
The team has also identified two FDA-approved drugs that can ease disease severity. The findings were published in Nature Biomedical Engineering, and a patent application has been filed based on the research.
Large-scale screening
The SARS-CoV-2 virus, which caused the worldwide COVID-19 pandemic, has been continuously evolving since its emergence. Spike protein mutations can lead to different degrees of infectivity and lethality. The spike protein plays a crucial role in viral infection by binding to human ACE2 receptors and merging with neighboring uninfected cells to form syncytia, which contribute to virus spread and disease severity.
Scientists have discovered that severe COVID-19 cases often exhibit syncytium cells in the lungs, providing evidence of the connection between spike-driven cell fusion and infection severity.
Large-scale screening to assess cell-cell fusion is challenging, as it requires a rapid way to measure the fusion process. Previous studies individually constructed and monitored spike variants to assess their potential to form syncytia, which was both technically demanding and costly.
To address this, the research team used innovative screening methods and advanced genetic techniques to identify the specific genetic and cellular factors responsible for promoting syncytia formation. This research is crucial for developing effective interventions and treatments to block the fusion and potentially restrict virus spread.
Two cellular factors: AP2M1 and FCHO2
The team used a special system involving split green fluorescent protein (GFP), which produces detectable signals when cells fuse together. They combined it with a microfluidics-based system and large-scale mutagenesis to create a new platform for rapid screening and analysis of a wide range of spike protein variants and their fusion capabilities.
By studying the spike protein and its mutations, researchers have found that certain variants, such as the delta strain, form larger syncytia than the original strain of the virus. They discovered that a single K854H mutation can transform the omicron variant into a strain that forms fusions from a low rate to a high rate.
Furthermore, some mutations found in the study were predicted to be as mutable as those seen in the previous variants, such as omicron and delta, revealing that these new mutations should be kept under surveillance as they could emerge in future viral evolution.
To enhance the efficiency of the screening process, the team developed a new strategy using size-exclusion selection to sort fused and unfused cells on a large scale, which enabled speedy screening. This method achieved more than 80% in accuracy rate, comparing with the traditional approach.
Employing the size-exclusion selection strategy, the team conducted a genome-wide CRISPR screen and identified two cellular factors, AP2M1 and FCHO2, involved in cellular uptake mechanism, which contributes to syncytium formation. The researchers then tested two FDA-approved drugs, chlorpromazine and fluvoxamine, currently used for antipsychotic purposes, to inhibit cellular uptake. Experiments on hamster models showed that these drugs could inhibit syncytium formation induced by the spike protein and potentially alleviate disease severity.
A potential therapeutic target
The significance of this study lies in its interdisciplinary approach, combining high-throughput CRISPR screening, large-scale mutagenesis, droplet microfluidics, and virology. The droplet microfluidics system enabled the study of cell-cell interactions in a high-throughput manner and provided high-resolution data.
The paired-cell system has the potential to systematically profile the fusogenicity of various viruses, including HIV, RSV, Herpesviridae, SARS-CoV-2 and other coronaviridae that induce syncytium formation.
The combination of high-throughput profiling systems and CRISPR screening led to the discovery of a potential therapeutic target to combat syncytium formation and reduce the severity of SARS-CoV-2 infections.
Professor Alan Wong Siu-lun, Associate Professor in the School of Biomedical Sciences, HKUMed, who led the research, stated, "These innovative systems enable us to rapidly track SARS-CoV-2 mutations on a larger scale and identify treatment options; they can also be broadly applied in the study of various pathological and physiological cell fusion conditions relevant to biomedical research, including cancer immunotherapy."
"The methods and knowledge gained from this study have the potential to contribute to public health efforts and provide insights into the development of therapeutic interventions for COVID-19 and other diseases involving cell fusion."
More information: Charles W. F. Chan et al, High-throughput screening of genetic and cellular drivers of syncytium formation induced by the spike protein of SARS-CoV-2, Nature Biomedical Engineering (2023). DOI: 10.1038/s41551-023-01140-z