This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Research team develops novel PTPN2/N1 inhibitor for cancer immunotherapy using generative AI
In recent years, cancer immunotherapy, exemplified by PD-1 and its ligand PD-L1 blockade, has made remarkable advances. But while immunotherapy drugs offer new treatment possibilities, only about 20% to 40% of patients respond to these treatments. The majority either don't respond or develop drug resistance. Researchers are now looking for ways to enhance the scope of tumor immunotherapy in order to benefit a wider range of patients.
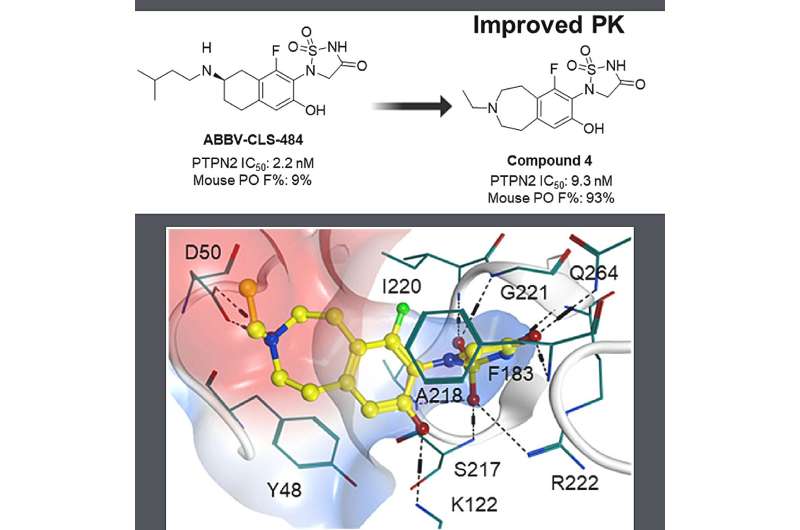
One such avenue is through the protein tyrosine phosphatase non-receptor type 2 (PTPN2) and its close superfamily member, PTPN1, identified in previous research as crucial modulators involved in the regulation of immune cells signaling pathways that promote tumorigenesis by attenuating tumor-directed immunity. While promising, the development of PTPN2/PTPN1 inhibitors has faced challenges as a result of unfavorable pharmacokinetics due to the highly cationic active site and the relatively shallow nature of the protein surface.
In a significant milestone, researchers at Abbvie discovered the dual PTPN2/N1 inhibitor ABBV-CLS-484 through structure-based drug design and optimization of drug-like properties. Now, clinical stage artificial intelligence (AI)-driven drug discovery company Insilico Medicine ("Insilico") has initiated a program with a fast-follow strategy to design a novel PTPN2/N1 inhibitor with drug-likeness properties and in vivo oral absorption, supported by the Company's generative AI drug design engine Chemistry42.
The research was published in the European Journal of Medicinal Chemistry.
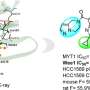
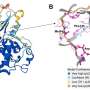
Scientists entered the structure of the known PTPN2/N1 inhibitor as a reference compound into Chemistry42 as a starting point and generated a series of novel PTPN2/N1 inhibitors based on ligand-based drug design strategy. They further optimized and synthesized the most promising molecules and obtained candidates with desirable ADME properties.
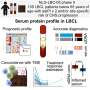
Insilico's compound demonstrated enhanced oral absorption, systemic exposure, and equivalent biological activities compared to the reference compound in in vitro studies. Furthermore, Insilico's compound demonstrated the same efficacious dose as the reference compound in a murine model.
"One of the most significant advances in the research was validating the fast follow ability of Chemistry42, the molecular generation and design engine of Pharma.AI, which allows users to improve existing molecules with more desirable properties rapidly," said Xiao Ding, Ph.D., vice president and head of medicinal chemistry of Insilico Medicine.
"In this paper, we reported a novel PTPN2/PTPN1 inhibitor demonstrating nanomolar inhibitory potency, good in vivo oral bioavailability, and robust in vivo antitumor efficacy. Further investigation is currently ongoing."
More information: Jiamin Zheng et al, Synthesis and structure-activity optimization of azepane-containing derivatives as PTPN2/PTPN1 inhibitors, European Journal of Medicinal Chemistry (2024). DOI: 10.1016/j.ejmech.2024.116390