This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
CAR T cell therapy for pediatric brain cancer warrants further study
Novel therapeutic approaches are needed for children with diffuse midline glioma (DMG) and other recurrent high-grade central nervous system (CNS) tumors, aggressive brain cancers with poor prognoses. A type of immunotherapy using T cells modified with chimeric antigen receptors (CAR T) has demonstrated efficacy in other cancers, but use in DMG and other CNS tumors has been limited.
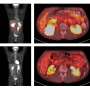
Researchers at Texas Children's Cancer Center and the Center for Cell and Gene Therapy at Baylor College of Medicine, Texas Children's Hospital and Houston Methodist Hospital have developed an augmented CAR T cell therapy targeting the GD2 antigen, which is highly expressed in DMG and other CNS tumors. The therapy was well tolerated in a phase 1 trial at Texas Children's Cancer Center; some patients showed temporary improvement of tumor-associated neurological deficits, warranting further investigation of this novel therapy.
The results are published in the Journal of Clinical Oncology.
"Brain tumors are very immunosuppressive and effective at turning CAR T cells off. We aimed to overcome that challenge by modifying the GD2 CAR T cells with a receptor called C7R," said senior author Dr. Bilal Omer, associate professor of pediatrics—hematology and oncology at Baylor, member of the Center for Cell and Gene Therapy and oncologist at Texas Children's Cancer Center. "This study shows that this therapy can effectively get to the tumor, and the addition of C7R safely augments antitumor activity."
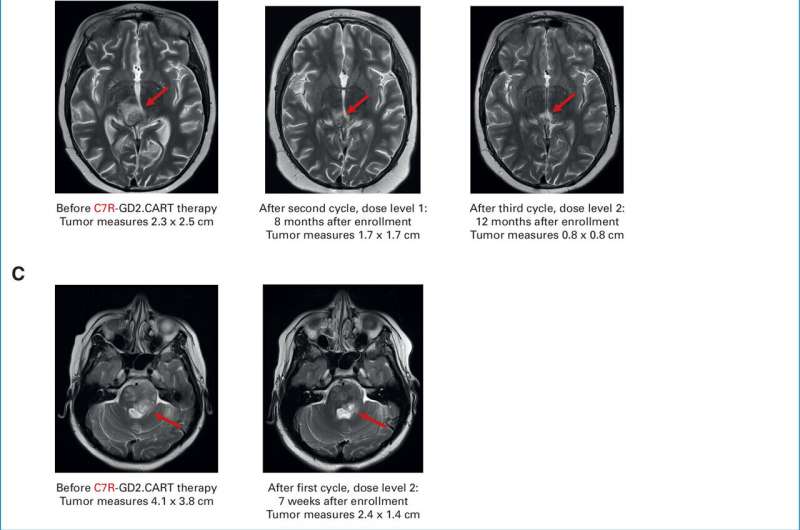
All patients received standard-of-care radiation therapy or chemotherapy prior to study enrollment. The first cohort received therapy targeting GD2 alone; patients in this group did not experience major side effects, but cancer progressed after a brief improvement of neurological symptoms.
Later cohorts received therapy enhanced with C7R, developed in Dr. Cliona Rooney's lab at the Center for Cell and Gene Therapy. Patients in this group experienced more initial side effects but also longer temporary improvement of neurological deficits, with a median improvement time of five months. A partial response was observed in two out of seven patients with DMG. One of these patients had a persistent response that is ongoing after more than two years.
"Seeing a signal from therapies for patients with these tumors is impactful and tells us we need to keep working in this direction," said first author Dr. Frank Lin, assistant professor of pediatrics—hematology and oncology at Baylor and neurooncologist at Texas Children's Cancer Center. "We benefit from the shared expertise of our multidisciplinary team to explore a cutting-edge therapy, with the goal of addressing a major unmet need for children with CNS cancers."
In the next arm of the study, researchers will examine whether the way the therapy is delivered, intravenously or directly into the spinal fluid, can impact the response to treatment. They will also study why some patients respond to treatment better and how the response to treatment time can be extended for all patients.
More information: Frank Y. Lin et al, Phase I Trial of GD2.CART Cells Augmented With Constitutive Interleukin-7 Receptor for Treatment of High-Grade Pediatric CNS Tumors, Journal of Clinical Oncology (2024). DOI: 10.1200/JCO.23.02019