This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New insights into the evolution of the prion protein could guide future neurodegenerative disease research
A study from Bochum describes a mammal-specific domain of the prion protein and offers new approaches for research into neurodegenerative diseases.
Prion diseases are progressive and invariably fatal neurodegenerative diseases. They are caused by misfolded prion proteins. At first, they cause memory deficits and difficulties in walking, finally they inhibit elementary motor skills and destroy basic brain functions. The mechanisms behind their development are still poorly understood.
Researchers at Ruhr University Bochum, Germany, have now discovered a new region in prion proteins and thus identified a potential new area for research into neurodegenerative diseases. This domain is specific to mammalian prion proteins and influences the aggregation of the protein.
The neuroscientists published their research results on April 22, 2024, in the Journal of Biological Chemistry.
Misfolded prion proteins disrupt the function of neurons in the brain
Prion diseases are very rare. About 2 people in 1 million are affected. One of the best-known examples is Creutzfeldt-Jakob disease, which gained particular prominence during the BSE crisis in the 1990s. As the disease progresses, the brain takes on a sponge-like perforated structure and shows protein deposits.
"The disease is triggered by the misfolding of the cellular prion protein, PrP for short, in the brain," explains Professor Jörg Tatzelt from the Institute of Biochemistry and Pathobiochemistry at Ruhr University Bochum, who led the study. "The pathological proteins accumulate in the brain and form aggregated deposits, so-called plaques. This gradually impairs the function of the neurons."
Prion diseases have so far only been confirmed in mammals. However, prion proteins are also found in the brains of frogs and reptiles. So why do they apparently not suffer from degenerative diseases?
"The brains of frogs and reptiles developed much earlier in evolutionary history than the brains of mammals," says first author Dr. Janine Kamps. "Our assumption was that the biophysical and biochemical properties of the prion proteins have changed in the course of evolution and that the toxic misfolding only occurs in mammals."
Indications of a new function of the prion protein in mammals
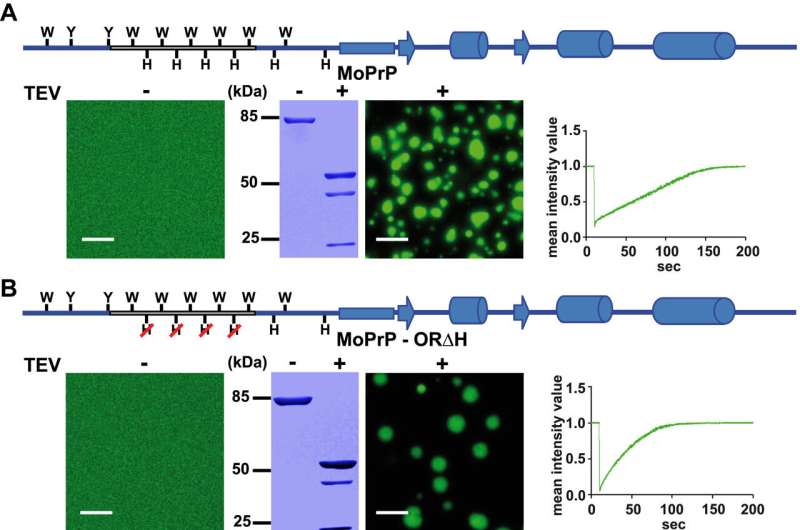
To test their hypothesis, the researchers analyzed the folding of purified prion proteins from frogs and mice using fluorescence microscopy. After their studies, they compared the results. "We were able to identify a protein region that only occurs in the PrP of mice, in other words in the PrP of mammals, and that influences protein folding and aggregation," explains Tatzelt, who is a Principal Investigator at the Research Department of Neuroscience at Ruhr University Bochum.
"The structure of prions typically shows two subdomains in their formation—one structured and one intrinsically disordered," adds Kamps. Interestingly, the disordered region, the so-called N-terminal domain of the prion protein, has developed differently in mammals than in amphibians. This suggests a new function for mammalian PrP that the amphibian protein does not yet have.
The results contribute to a better understanding of the mechanisms of protein folding and could help to develop treatment approaches for prion diseases in the long term. The neuroscientists' next research project will build directly on the discoveries made.
Tatzelt says, "We will now investigate to what extent the mammalian-specific domain is associated with the ability of PrP to trigger neurodegenerative diseases."
More information: Janine Kamps et al, Liquid–liquid phase separation of the prion protein is regulated by the octarepeat domain independently of histidines and copper, Journal of Biological Chemistry (2024). DOI: 10.1016/j.jbc.2024.107310