This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Sanfilippo syndrome: Research team resolves structure of crucial enzyme for the first time
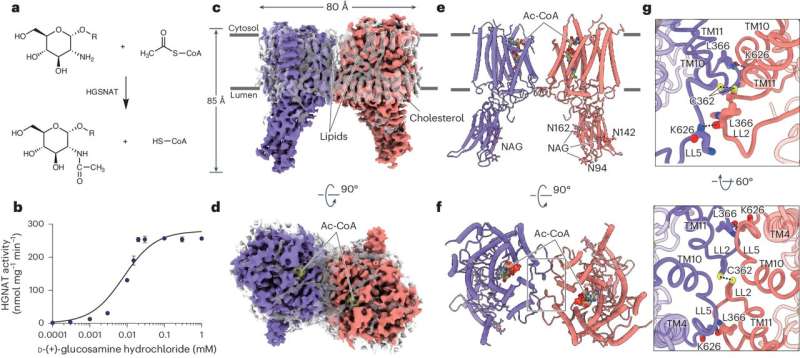
For the first time, a team co-led by CHU Sainte-Justine researcher and professor in the Faculty of Medicine at Université de Montréal, Alexey Pshezhetsky has succeeded in resolving the unique structure of the HGSNAT enzyme, a deficiency of which causes Sanfilippo syndrome, a rare pediatric disease affecting the central nervous system.
Through collaboration with a team from Shanghai University, the structure of this enzyme and the mechanism of its function were revealed using high-performance cryogenic electron microscopy. This discovery will enable the development of new treatments for Sanfilippo disease as well as similar fatal neurodegenerative disorders.
The study is published in the journal Nature Structural & Molecular Biology.
A deadly and complex condition
Sanfilippo syndrome is a metabolic disorder resulting from genetic mutations that cause the accumulation of the molecule heparan sulfate in brain cells. The HGSNAT enzyme is essential for the degradation of heparan sulfate. In Sanfilippo syndrome, genetic mutations in HGSNAT prevent it from folding and functioning correctly. As a result, heparan sulfate accumulates, causing neuronal death and leading to dementia in affected children.
"In basic terms, Sanfilippo is similar to Alzheimer's disease, but it occurs in children aged 2 or 3 years old," explained researcher Alexey Pshezhetsky, who is also a professor at Université de Montréal. "Starting from this age, the affected children's development stagnates, and they then regress, eventually passing away toward the end of adolescence."
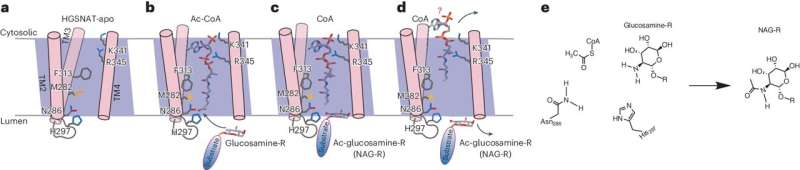
Although there is currently no treatment for this condition, much hope is pinned on what is called "chaperone therapy" (CT). CT involves administering a small molecule that binds to the mutant enzyme and helps it fold properly, enabling it to function normally.
To determine which molecule is best suited for binding to the enzyme, a reliable model of its structure is required—which the team has successfully developed using cryogenic electron microscopy.
Paving the way for new treatments
As the Elisa Linton Research Chair in Lysosomal Diseases, Professor Alexey Pshezhetsky is actively working to translate this new knowledge into clinical applications. "In collaboration with Professor Christopher Cairo from the University of Alberta, we are currently determining which small molecules bind best to the enzyme and, therefore, would be most effective in treating the disease," he added.
Discussions are already underway with other Canadian researchers to study treatments that combine CT with gene therapy or stem cell-based therapy. "Confidence in the effectiveness of such combined approaches is currently growing, and we are certain that over the next few years, a new therapeutic strategy will be developed to benefit affected children and their families," he concluded.
More information: Ruisheng Xu et al, Structure and mechanism of lysosome transmembrane acetylation by HGSNAT, Nature Structural & Molecular Biology (2024). DOI: 10.1038/s41594-024-01315-5

















