This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
Advancements in protein engineering offer hope for cancer therapies
Cancer is one of the most devastating diagnoses that a person can receive, and it is a society-wide problem. According to the National Cancer Institute, an estimated 2,001,140 new cases of cancer will be diagnosed in the United States and 611,720 people will die from the disease this year.
While cancer treatments have seen large improvements over the last decades, researchers are still laser-focused on developing strategies to defeat cancers, especially those that have been resistant to traditional interventions.
New research from Jin Kim Montclare, Professor of Chemical and Biomolecular Engineering, may lend hope to cancer patients in the future. Montclare's lab, which uses customized artificial proteins to target human disorders, drug delivery and tissue regeneration utilizing a blend of chemistry and genetic engineering, has recently published two papers that take aim at cancers that have been difficult to treat.
MAPping a course to recovery
In the ongoing quest to develop more effective cancer therapies, these researchers have unveiled a promising approach utilizing protein-based targeting agents. A study published in Biomaterials Science introduces a novel strategy that harnesses the power of multivalent assembled proteins (MAPs) to target hypoxic tumors with unprecedented precision and efficacy.
Traditional cancer treatments often rely on passive or active targeting mechanisms to deliver therapeutic agents to tumor sites. However, these approaches have limitations, particularly in overcoming physiological and pathological barriers. To address these challenges, the research team focused on exploiting the unique features of the tumor microenvironment (TME), specifically its hypoxic conditions.
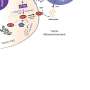
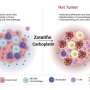
Hypoxia, a characteristic feature of many solid tumors, is known to play a crucial role in tumor progression and resistance to therapy. By targeting hypoxia-inducible factor 1 alpha (HIF1α), a key regulator of cellular response to low oxygen levels, the researchers aimed to develop a more effective strategy for tumor-specific drug delivery.
Previous efforts to target HIF1α have been hindered by the instability and limited binding abilities of the peptide-based molecules used. To overcome these obstacles, the team turned to MAPs, which offer the advantages of high stability and multivalency.
Drawing inspiration from their successful development of MAPs targeting COVID-19, the researchers engineered HIF1α-MAPs (H-MAPs) by grafting critical residues of HIF1α onto the MAP scaffold. This innovative design resulted in H-MAPs with picomolar binding affinities, significantly surpassing previous approaches. In vivo studies showed promising results, with H-MAPs effectively homing in on hypoxic tumors.
"This is a very promising result, using a material that is degradable within the body and likely will limit side effects to treatments," said Montclare. "We're taking advantage of the building blocks of our own bodies and using those protein compositions to treat the body—and that's where we're making big progress."
Montclare's findings suggest that H-MAPs hold great potential as targeted therapeutic agents for cancer treatment. With further refinement and exploration, H-MAPs could offer a new avenue for precision medicine, providing clinicians with a powerful tool to combat cancer while minimizing side effects and maximizing therapeutic efficacy.
The development of H-MAPs represents a significant advancement in the field of cancer therapy, highlighting the importance of innovative approaches that leverage the intricacies of the tumor microenvironment. As researchers continue to unravel the complexities of cancer biology, protein-based targeting agents like H-MAPs offer hope for improved outcomes and better quality of life for cancer patients.
Targeting stubborn breast cancer subtypes
Not all tumors are hypoxic, however, and some forms of cancers are much harder to target than others.
Triple-negative breast cancer (TNBC) poses a significant challenge in the realm of oncology due to its resistance to conventional targeted therapies.
Unlike other breast cancer subtypes, TNBC lacks the receptor biomarkers—such as estrogen receptors and human epidermal growth factor receptor 2 —making it unresponsive to standard treatments. Consequently, chemotherapy remains the primary option for TNBC patients. However, the efficacy of chemotherapy is often hindered by the development of drug resistance, necessitating innovative approaches to enhance treatment outcomes.
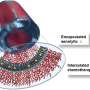
In recent years, there has been a surge of interest in improving the efficacy of chemotherapy for TNBC through enhanced drug delivery systems. One promising avenue involves the use of biocompatible materials, including lipids, polymers, and proteins, as carriers to encapsulate chemotherapeutic agents. Among these materials, protein-based hydrogels have emerged as a particularly attractive option due to their biocompatibility, tunable properties, and ability to achieve controlled drug release.
A recent breakthrough in the field comes from the development of a novel protein-based hydrogel, known as Q8, which demonstrates remarkable improvements over previous materials
By fine-tuning the molecular characteristics of the hydrogel using a machine learning algorithm, the researchers were able to engineer Q8 to exhibit a two-fold increase in gelation rate and mechanical strength. These enhancements pave the way for Q8 to serve as a promising platform for sustained chemotherapeutic delivery.
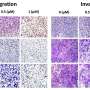
In a study, published in ACS Biomaterials Science & Engineering, the researchers investigated the therapeutic potential of Q8 for the treatment of TNBC in vivo using a mouse model. Remarkably, the delivery of doxorubicin encapsulated in Q8 resulted in significantly improved tumor suppression compared to conventional doxorubicin treatment alone.
This achievement marks a significant milestone in the development of non-invasive and targeted therapies for TNBC, offering new hope for patients facing this aggressive form of breast cancer.
The success of Q8 underscores the immense potential of protein-based hydrogels as versatile platforms for drug delivery in cancer therapy. By leveraging the unique properties of these materials, researchers can overcome longstanding challenges associated with chemotherapy, including poor drug bioavailability and resistance.
Moving forward, further advancements in protein engineering and hydrogel design hold the promise of revolutionizing cancer treatment paradigms, offering renewed optimism for patients battling TNBC and other challenging malignancies.
More information: Dustin Britton et al, Coiled-Coil Protein Hydrogels Engineered with Minimized Fiber Diameters for Sustained Release of Doxorubicin in Triple-Negative Breast Cancer, ACS Biomaterials Science & Engineering (2024). DOI: 10.1021/acsbiomaterials.4c00349
Dustin Britton et al, Engineered coiled-coil HIF1α protein domain mimic, Biomaterials Science (2024). DOI: 10.1039/D4BM00354C