This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
proofread
Researchers evaluate the benefit of dual therapy for children at risk for spinal muscular atrophy
In a first-of-its-kind study, researchers compared the efficacy of preventative therapy for spinal muscular atrophy (SMA) between two well-matched study groups, using either gene therapy (onasemnogene abeparvovec) alone or in combination with risdiplam (oral medication) or nusinersen (intrathecal injection) administered before apparent signs of disease emerged.
The study included presymptomatic infants with two or three copies of SMN2 at risk for developing SMA type 1 or 2, respectively. SMA is a devastating rare genetic disorder that leads to progressive degeneration of spinal motor neurons that control movement, swallowing, and breathing. The paper was recently published by researchers from the Clinic for Special Children and Children's Hospital of Philadelphia in the Annals of Clinical and Translational Neurology.
The study followed twenty-three (23) infants who received preventative therapy within six weeks of age, before the onset of visible weakness. They were divided into three groups: babies with two copies of SMN2 (SMA type 1) who received only gene therapy, babies with two copies of SMN2 (SMA type 1) who received gene therapy plus risdiplam or nusinersen, and babies with three copies of SMN2 (SMA type 2) who received only gene therapy. The decision to add a second therapy was made between parents and caregivers in the context of routine clinical management.
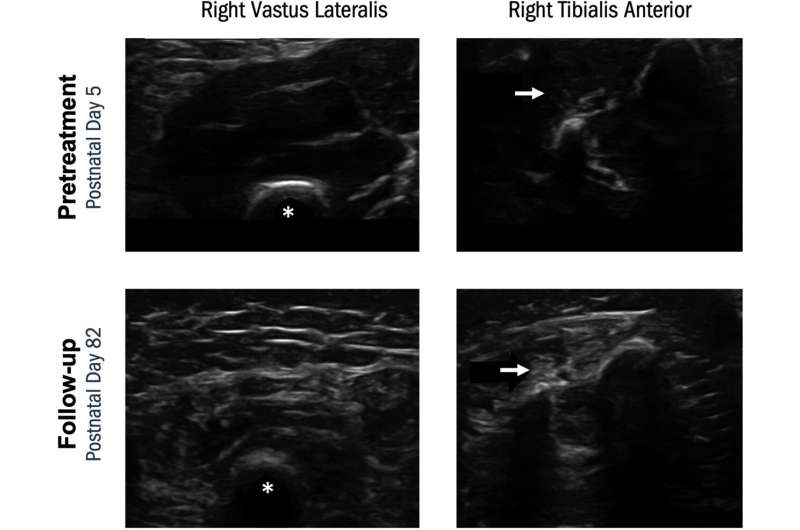
Researchers assessed the effectiveness of single vs. dual therapy by recording independent sitting and walking outcomes and conducting muscle ultrasounds and nerve conduction studies. Children on dual therapy sat sooner but did not appear to walk at an earlier age than those on monotherapy. Furthermore, abnormal skeletal muscle ultrasound demonstrated abnormal fat infiltration and fasciculations in both the monotherapy and dual therapy cohorts, while children with three copies of SMN2 (SMA type 2) had largely normal muscle ultrasounds.
The results showed that while dual therapy was well tolerated, the additional therapy did not prevent widespread muscle disease progression. Long-term studies of developmental outcomes in these children are warranted.
"This study is the first to compare children receiving early monotherapy or dual therapy in SMA, addressing an important question for the patient community in a real-world setting. We will continue to follow these children over time to determine if and how combination therapy improves functional outcomes in patients at risk for type 1 SMA to best guide clinical management for them," summarizes Karlla W. Brigatti, Research Director at the Clinic for Special Children.
More information: Susan E. Matesanz et al, Preemptive dual therapy for children at risk for infantile‐onset spinal muscular atrophy, Annals of Clinical and Translational Neurology (2024). DOI: 10.1002/acn3.52093