This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Discovery unveils key to heart development in womb, unravels cause of spongy heart disease
Having explored how the heart is formed in utero, a University of Houston pharmacology researcher is reporting how cells and molecules act during that early formation and what might cause the heart disease called left ventricular non-compaction. LVNC is a type of heart muscle disease (or cardiomyopathy) also known as a spongy heart, for which patients often require heart transplants.
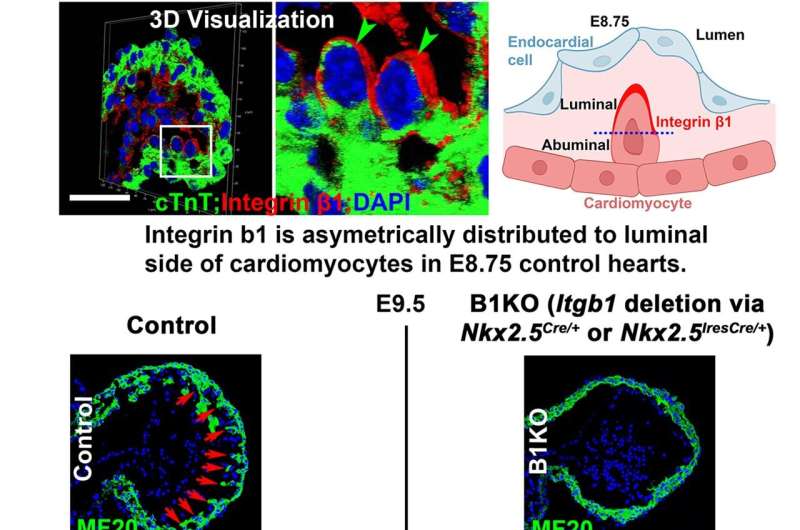
"We found the absence of a certain gene, called Itgb1, may cause inability in the developing heart to maintain its shape and develop normally, causing left ventricular non-compaction," reports Mingfu Wu, associate professor at the UH College of Pharmacy. "Itgb1 deletion at an early stage causes distinct defects."
The study is published in the journal Cardiovascular Research.
Wu's findings involve examination of the trabeculae, which are sheet-like structures in developing hearts that protrude from the heart wall. These structures are crucial in early heart development—before the coronary system develops—to supply blood, as they increase the inner surface area of the heart wall and enhance the exchange of oxygen and nutrients between blood and the heart wall.
"Without trabeculae, the heart wall would suffer from insufficient oxygen and nutrients, potentially leading to death. Conversely, an excess of trabeculae can result in an overly porous heart wall, leading to left ventricular non-compaction cardiomyopathy, commonly known as spongy heart," said Wu.
While certain signals controlling trabeculae formation are known, the precise mechanisms by which individual cells that make up the cardiac muscle (cardiomyocytes) are organized to form these structures remain unclear.
"This study reveals that deleting a gene called Itgb1 in heart wall cardiomyocytes prevents trabeculae formation. We found that the protein β1 integrin encoded by Itgb1 and its ligands, the extracellular matrix, create a molecular network that acts as a scaffold for cardiomyocytes in the heart wall. When Itgb1 is deleted, cardiomyocytes disengage from this scaffold, losing their ability to maintain shape, divide properly, migrate, and form trabeculae," said Wu.
The study suggests that the molecular network may be a common organizational mechanism in organ formation.
"We believe these insights will greatly interest researchers in organ development and regeneration, potentially reshaping our understanding of tissue organization and development," said Wu.
Wu's collaborators from the University of Houston include Lianjie Miao, Yangyang Lu, Anika Nusrat, Luqi Zhao Micah Castillo, Yongqi Xiao, Hongyang Guo, Yu Liu, Preethi Gunaratne, Robert J. Schwartz, Alan R. Burns, Ashok Kumar and C. Michael DiPersio, Albany Medical College.
More information: Lianjie Miao et al, β1 integrins regulate cellular behaviour and cardiomyocyte organization during ventricular wall formation, Cardiovascular Research (2024). DOI: 10.1093/cvr/cvae111