This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
New drug benefits patients with Sjögren's disease in phase 2 clinical trial
In a phase 2 clinical trial, a new drug under development significantly reduced both the symptoms and disease process of a condition called Sjӧgren's disease (SjD).
SJD is marked by eye and mouth dryness, fatigue, and pain and often occurs in association with rheumatoid arthritis and systemic lupus, but it can also be its own primary disease.
The international study was led by E. William St. Clair, M.D., the W. Lester Brooks Jr. Professor of Medicine in the Division of Rheumatology and Immunology at Duke University School of Medicine. Results were published June 5 in the journal Nature Medicine.
"This is hopeful news for people with Sjögren's," St. Clair said. "There are currently no disease-modifying therapies for SjD, so current treatment is usually aimed at reducing symptoms."
Among the most common autoimmune diseases, SjD affects mostly women. It typically develops in middle-age but can affect people of all ages. Systemic disease can cause inflammation, leading to arthritis, skin rashes, lung disease, kidney disease, nervous system disease, and vasculitis.
A new drug—Dazodalibep or DAZ—blocks the signals that drive autoimmunity in SjD. In this phase study, two different study populations of SjD patients were enrolled and randomly assigned to either receive DAZ therapy or a placebo drug.
The first group of participants included 74 patients with moderate to severe systemic disease activity, and the second group consisted of 109 patients with severe symptoms but limited serious organ system involvement.
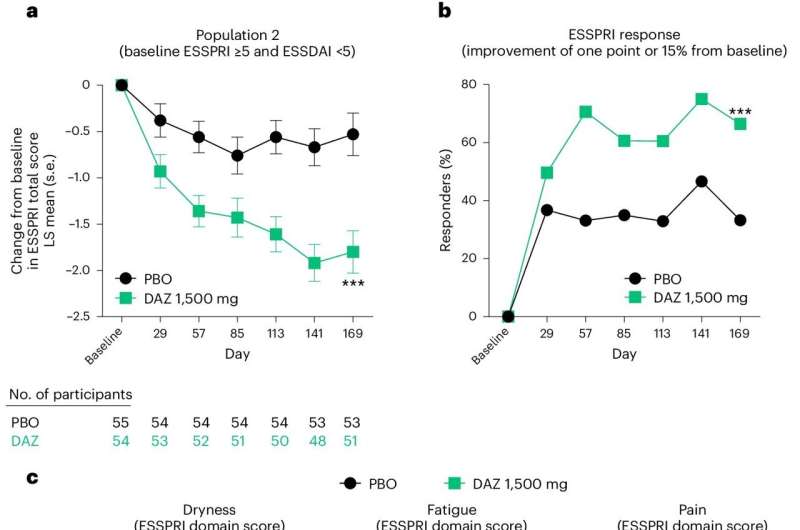
After 169 days, the moderate to severe group showed DAZ therapy produced significant improvement using a multi-dimensional index measuring systemic disease activity. In the second population, DAZ therapy also reduced symptoms over the same timeframe using a scale that evaluates the degree of dryness, fatigue, and pain.
"DAZ is the first new drug under development for the treatment of SjD to reduce both systemic disease activity and an unacceptable symptom burden," St. Clair said. "These findings need confirmation in larger clinical trials."
More information: E. William St. Clair et al, CD40 ligand antagonist dazodalibep in Sjögren's disease: a randomized, double-blinded, placebo-controlled, phase 2 trial, Nature Medicine (2024). DOI: 10.1038/s41591-024-03009-3