This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
trusted source
proofread
Gaining a better understanding of brittle bone disease with 3D model featuring biodegradable hydrogel matrix
For someone suffering from brittle bone disease, life is fraught with complications. The slightest misstep, a seemingly harmless fall or even one false move can be all it takes to leave them with a broken arm or leg. And chances are this will happen repeatedly, because they were born with an inherited genetic defect that makes their bones extremely brittle and is often associated with physical deformity. Notable sufferers of brittle bone disease include German author and actor Peter Radtke and French jazz pianist Michel Petrucciani.
In most cases, what causes a person to have brittle bones is a mutation in the gene that carries the blueprint for the type I collagen protein. This is by far the most important protein for establishing a hard bone matrix.
People with this condition have a genetic defect that prevents this collagen protein from folding correctly, leaving them with an unstable bone matrix and brittle bones. The proper name for brittle bone disease is osteogenesis imperfecta, or OI for short.
A porous matrix structure
So far, scientists have had only a rudimentary understanding of how mutations in the collagen protein disrupt the formation of the bone matrix, as well as of how to go about treating these malformations. But now, a group of researchers at ETH Zurich's Institute for Biomechanics has taken a major step towards answering these questions.
Leading the team is Xiao-Hua Qin, Professor of Biomaterials Engineering, in collaboration with fellow ETH Professor Ralph Müller. Together, they have developed a 3D in vitro model that allows them to investigate bone formation in greater detail—currently using healthy cells and in the future also using cells from people afflicted with OI.
The researchers report on their progress in the journal Nature Communications.
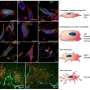
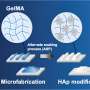
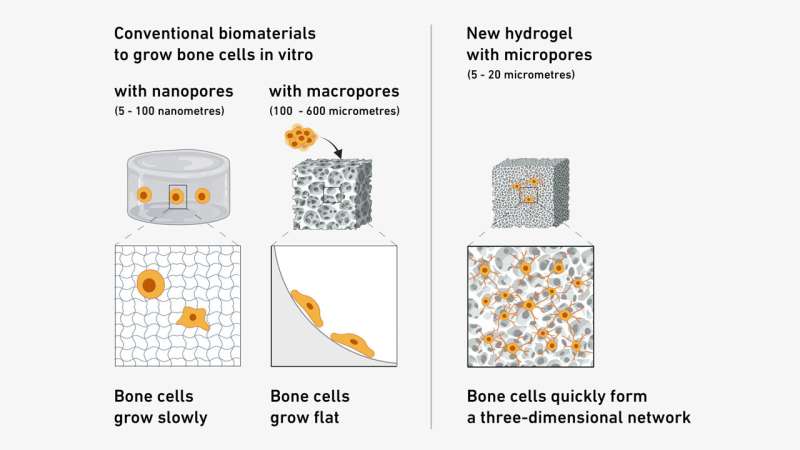
This new bone model is based on a porous matrix, or structure, made of a synthetic polymer. In this matrix, made of a soft hydrogel, the cells (osteoblasts) that form bone can settle, multiply and connect with each other and their offshoots to form a three-dimensional network.
During development, the researchers ascertained that the ideal pore size is between 5 and 20 micrometers: wide enough to allow the cells to settle and multiply, yet narrow enough to prevent them from escaping.
In creating their hydrogel, the researchers took their cue from in vitro models for nerve cells. "Porous hydrogels provide neurons with an extremely conducive environment in which to form artificial networks," Qin says.
It soon became clear, however, that bone precursor cells "react completely differently" in one respect: while they also require a porous matrix, this matrix must be biodegradable. So the researchers equipped their hydrogel with what's known as a peptide crosslinker, which can be broken down by a matrix metalloproteinase (MMP) enzyme. This in turn enables the cells to produce more mature collagen fibers. MMPs are essential for many bodily processes, one of them being bone formation.
To ensure that the bone cells could grow and network correctly, however, the researchers had to first solve yet another problem.
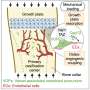
"Studying bone development, as well as bone remodeling, involves mechanically stimulating the cells," says Doris Zauchner, a doctoral student in Qin's group and lead author of the research paper. The researchers placed a hydrogel with embedded cells onto a chip and channeled a liquid through the pores.
"This liquid subjects the cells to shearing forces," Zauchner says, which is important for cell function. A liquid carrying nutrients and chemical messengers has also been shown to mechanically stimulate cells in the pores of healthy bones.
Model closely resembles normal bone formation
As the researchers describe in their paper, their bone model featuring the biodegradable hydrogel matrix and mechanical stimulation can successfully emulate bone development. The osteoblasts reproduce and, in some cases, even develop into immature osteocytes (which account for 90% of the cells in healthy bones); they secrete collagen and can mineralize the matrix.
"It might be just a model," Zauchner says, "but it quite closely resembles normal bone development." Now that they have patented their model, the researchers plan to make it available to potential industry partners.
Compared to previous bone formation models, the new in vitro model on the chip offers numerous advantages. The pores in these predecessor models were either too narrow, so that the cells barely had room to maneuver, or too wide, so that no three-dimensional network could form.
Moreover, as these models used collagen for their matrix structure, it was impossible to study whether it was the cells themselves producing collagen, and if so, how much. Because the model is small enough to fit on a chip, researchers can use it even if they only have a few cells from a patient at their disposal.
Replacing animal experiments
Up to now, the main means of researching OI has been to rely on animal models. Zauchner notes that there are more than 20 different ones, some using mice, others using fish or even dogs. "Animal experiments come with a host of constraints," she says, the main one being that they are extremely expensive.
"That's why we're trying to create an in vitro model for OI. Our goal is to embed cells from people with OI into the hydrogel with a view to discovering which processes are malfunctioning." Zauchner is poised to start the first experiments using cells from a young OI patient at the Children's Hospital Zurich.
The OI project is part of the Swiss National Research Program "Advancing 3R". Its overarching aim is to explore how to bring the 3R approach of Replace, Reduce and Refine to animal experiments.
Qin's group is looking to gain a better overall understanding of the processes governing how bones form, develop and degrade. To this end, the group's activities are not limited to the new OI model. In another project, for which Qin was recently awarded a prestigious ERC Starting Grant, the team is developing a model for degenerative bone diseases such as osteoporosis. The focus here is on osteocytes, which are terminally differentiated bone cells.
"We want to use the three-dimensional networks of human cells to build an in vitro model for bone development," Qin says. No one has managed this yet—except perhaps his own team. "In the model we've just unveiled, we found immature osteocytes as well as osteoblasts."
More information: Doris Zauchner et al, Synthetic biodegradable microporous hydrogels for in vitro 3D culture of functional human bone cell networks, Nature Communications (2024). DOI: 10.1038/s41467-024-49280-3