This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
How tumor stiffness alters immune cell behavior to escape destruction
Immunotherapy is based on harnessing a person's own immune system to attack cancer cells. However, patients with certain tumors do not respond to these therapies and it remains unclear why.
"The full impact of anti-cancer immunotherapy has not been realized, especially for some solid tumors," says Kevin Tharp, Ph.D., assistant professor in the Cancer Metabolism and Microenvironment Program at Sanford Burnham Prebys.
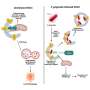
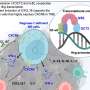
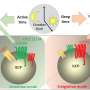
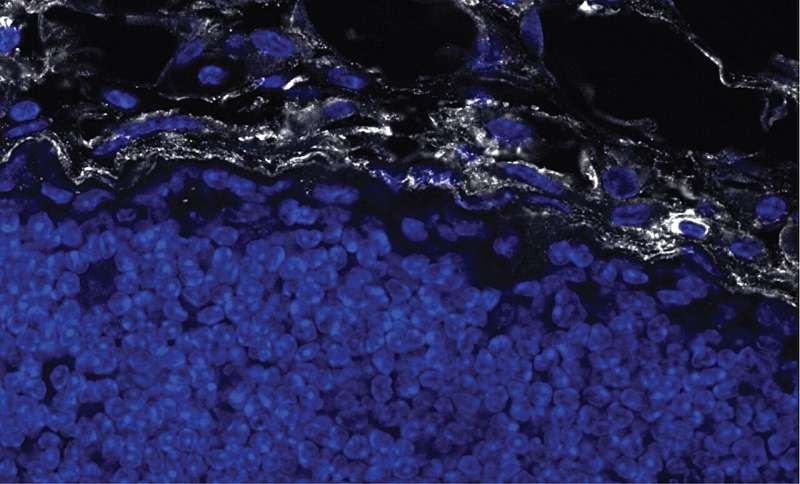
Researchers presume that part of the reason why these therapies fail is due to tumor-associated fibrosis, the creation of a thick layer of fibrous collagen (like scar tissue) that acts as a barrier to infiltrating anti-tumor immune cells such as cytotoxic T lymphocytes (CTLs).
In a new paper, published in Nature Cancer, first author Tharp and colleagues illuminate how the fibrotic tumor microenvironment creates an inhospitable milieu for anti-tumor immunity.
Kelly Kersten, Ph.D., an assistant professor who is also a member of the Cancer Metabolism and Microenvironment at Sanford Burnham Prebys, is a co-author on the paper. The senior author is Valerie M. Weaver, Ph.D., professor of surgery at University of California San Francisco where the research was primarily conducted.
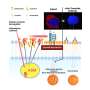
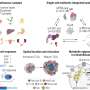
In the new study, which focused on breast cancer, the authors report that tumor-associated macrophages (TAMs), a type of immune cell found abundantly in the tumor microenvironment, respond to the physical properties of fibrosis by synthesizing injury-associated collagens that facilitate wound closure and "re-epithelialization."
The resulting metabolic changes in TAMs result in metabolic byproducts that suppress the anti-tumor function of CTLs.
Tharp said the metabolic changes in the microenvironment present more of a challenge to anti-tumor responses than the physical barrier.
"CTLs normally migrate through spaces much smaller than the gaps in collagen networks that form around tumors," Tharp says. "Our study provides an alternative explanation for why anti-tumor immunity is impaired in fibrotic solid tumors."
Kersten said the findings underscore that the phenotype and function of immune cells in the context of cancer are heavily regulated by environmental factors, such as tissue stiffness and metabolic challenges.
"Our findings help improve understanding of the mechanisms that regulate anti-tumor immune responses in fibrotic tumors and will aid the development of novel strategies to work in conjunction with immunotherapies to treat cancer patients."
Additional authors on the study include Ori Maller, Greg A. Timblin, Stashko Connor, Mary-Kate Hayward, Ilona Berestjuk, Bushra Samad and Alexis J. Combes, all at UCSF; Fernando P. Canale and Roger Geiger, Università della Svizzera italiana, Switzerland; Rosa E. Menjivar and Marina Pasca di Magliano, University of Michigan; Johanna ten Hoeve and Alastair J. Ironside, UCLA; and Alexander Muir, University of Chicago.
More information: Kevin M. Tharp et al, Tumor-associated macrophages restrict CD8+ T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment, Nature Cancer (2024). DOI: 10.1038/s43018-024-00775-4