This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
proofread
New imaging technique reveals intracellular energy dynamics in kidney cells
The prevalence of kidney disease has been increasing in Japan, with it now affecting one in eight adults, but developing effective treatment remains a challenge. The kidneys are among the most energy-intensive organs in the body.
For the kidneys to function, they constantly produce and consume large amounts of adenosine triphosphate (ATP), which is a chemical that the body uses to store and transport energy. However, ATP dynamics—the changes over time in ATP production and utilization—within the kidney have been poorly understood because of the lack of suitable imaging technologies.
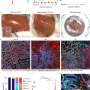
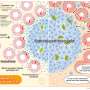
Using a newly developed ATP imaging system, researchers were able to visualize the amounts of ATP in various kidney cells, including deeper segments of nephrons, which are functional units within the kidney. This provides a detailed look at how energy is generated and used up in different parts of the kidney.
The study is published in the journal Kidney International.
This new system allowed the researchers to study ATP dynamics in real time using kidney slices taken from GO-ATeam2 mice, a genetically-modified mouse model recently developed by the researchers that expresses an ATP biosensor.
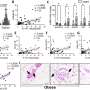
A key finding of the study was that there were distinct ATP synthesis pathways in different nephron segments. The proximal tubules were found to be highly dependent on oxidative phosphorylation (OXPHOS) for ATP production, while podocytes relied on both OXPHOS and the conversion of glucose. This ATP production in specific segments suggests that targeting these pathways could lead to more effective treatments for kidney diseases.
The researchers also used their imaging system to study ATP dynamics in disease models, including ischemia reperfusion injury and chemotherapy-induced damage. They found that ATP levels in proximal tubules were particularly affected in these models, highlighting the importance of energy metabolism in kidney injury.
Lead researcher Dr. Shigenori Yamamoto emphasized the importance of understanding the complex interactions among kidney cells when developing therapeutic strategies to improve kidney function. "Experimental techniques that allow for the analysis of multiple cell functions over time, including our novel system, will be a powerful tool," he notes.
The research team plans to further refine their imaging technique and use it to study ATP dynamics in various models of kidney injury, including those related to diabetes, aging, and drug-induced damage. By gaining a better understanding of how ATP production is affected in these conditions, they hope to identify new therapeutic targets and improve treatments for kidney diseases.
More information: Shigenori Yamamoto et al, Visualization of intracellular ATP dynamics in different nephron segments under pathophysiological conditions using the kidney slice culture system, Kidney International (2024). DOI: 10.1016/j.kint.2024.05.028