This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Preclinical study shows promise of systemic targeted therapy in a rare form of cancer
Pseudomyxoma peritonei is a rare and poorly understood cancer, characterized by the progressive accumulation of mucin in the abdominal cavity. Mainstay treatments are surgery and chemotherapy but disease recurrence and death after relapse frequently occur. The development of new, more effective therapeutic strategies represents an unmet clinical need.
Results of a preclinical study, published in Clinical Cancer Research, led by investigators of the Vall d'Hebron Institute of Oncology's (VHIO) Stem Cells and Cancer Group, headed by Héctor G. Palmer, pave the way for systemic targeted therapy in patients with pseudomyxoma peritonei, a rare form of cancer with very few therapeutic options available. This work was carried out in collaboration with colleagues at the San Joan Despí Moises Broggi Hospital, Barcelona.
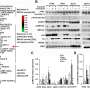
The investigators generated the largest collection of in vitro and in vivo patient-derived mouse models of pseudomyxoma peritonei with identified KRAS and BRAF druggable targets to guide the selection of molecular targeted therapies.
As a proof-of-concept, they studied the therapeutic efficacy of the BRAF inhibitor encorafenib in these preclinical models. Patients presenting these mutations—accounting for about 4%–8% of cases—have a poor prognosis. The researchers observed that treatment with encorafenib significantly reduced tumor growth and prolonged survival in mice.
"Results of this proof-of-concept study represent an important first step toward developing and applying systemic targeted therapy in the clinic for patients who could for the first time derive benefit from personalized, molecularly matched treatments.
"Currently, cytoreductive surgery is the mainstay of treatment, but many patients develop early recurrence and ultimately succumb to disease progression. There is an urgent medical need to provide new therapeutic strategies to more effectively combat this disease, " says Héctor G. Palmer, senior author of this present study.
Pseudomyxoma peritonei is a poorly understood cancer that usually starts in the appendix with an incidence of 1 to 3 cases per million per year. While rare, this disease is more likely to be diagnosed in people aged 40 years or over.
"We have generated the world's largest collection of patient-derived organoids and xenografts from patients with pseudomyxoma peritonei and showed that they are robust preclinical models to study this disease. To do so, we processed a total of 120 samples from 50 patients," observes Jordi Martínez -Quintanilla, Senior Investigator of Palmer's group and co-first author of this study along with Débora Cabot, a Laboratory Technician of the same group.
Unmasking druggable mutations: The genomic characterization of preclinical models and intra-abdominal mucin biopsy
For the first time, the investigators used intra-abdominal mucin biopsy to detect circulating tumor DNA (ctDNA) derived from cancer cells. They then identified those preclinical models presenting druggable mutations and observed that 80% of their preclinical models presented KRAS or BRAF mutations.
"While mutations in the KRAS gene were a lot more frequent, we decided to evaluate the efficacy of BRAF inhibitor encorafenib in our BRAFV600E models. BRAF inhibitors have revolutionized the treatment of BRAF-mutated metastatic colorectal cancer or melanoma, while KRAS inhibitors are currently in clinical phase development.
"We therefore believe that BRAF inhibition will be the most rapid option of molecularly matched therapy in this patient population, particularly considering that encorafenib monotherapy has already been approved for the treatment of other tumor types," explains Cabot.
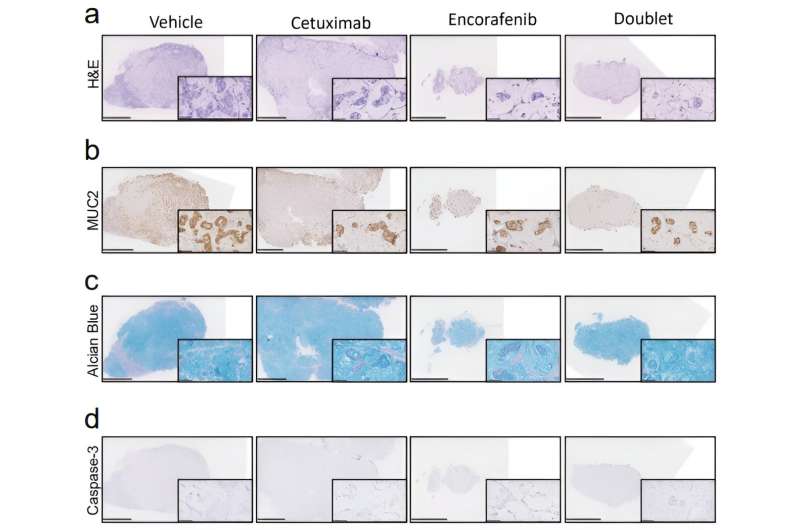
Organoid cultures were derived from high-grade BRAFV600E-mutated pseudomyxoma peritonei patient samples and tumors were generated in mice. The investigators observed that treatment with encorafenib slowed tumor growth in all cases.
"For the first time, we have shown that systemic targeted therapy for pseudomyxoma peritonei can effectively control tumor growth in animal models. BRAF inhibition could represent a new therapeutic opportunity for patients with BRAF-mutated disease who have a poor prognosis.
"Our data show promise in extending precision oncology to these patients, who could for the first time derive benefit from personalized matched targeted therapies," concludes Palmer.
The next step will be to validate these data in other models of BRAF-mutated pseudomyxoma peritonei to confirm whether KRAS inhibitors, currently being investigated in clinical trials, exhibit the same systemic antitumor activity in animal models.
More information: Jordi Martínez-Quintanilla et al, Precision oncology and systemic targeted therapy in Pseudomyxoma Peritonei, Clinical Cancer Research (2024). DOI: 10.1158/1078-0432.CCR-23-4072