This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
trusted source
proofread
Creating the next generation of mRNA vaccines: Study shows potential for lower-doses, longer-lasting protection
During the COVID-19 pandemic, mRNA vaccines came to the rescue, developed in record time and saving lives worldwide. Researchers in the Precision Vaccines Program at Boston Children's Hospital have developed two novel technologies that could make these and future mRNA vaccines more potent and longer-lasting—at smaller doses and with fewer side effects.
The mRNA COVID-19 vaccines currently used instruct cells to make the SARS-CoV-2 spike protein. This helps the immune system recognize the virus and quickly make antibodies against it. However, these vaccines offer only short-lived immune protection, requiring frequent boosters, and work poorly in people over 60. They also induce an inflammatory reaction throughout the body, causing side effects.
The lab of David Dowling, Ph.D., sought something better. "In current mRNA vaccines, delivery is not controlled," Dowling says. "Immunomodulation is kind of random and not built into the vaccine. We wanted to solve both of those problems through rational design."
Turbocharging vaccine responses
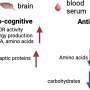
Dowling's lab has long studied a naturally occurring immune protein called interleukin-12 (IL-12). In 2012, the lab showed that IL-12 potently activates dendritic cells, crucial first responders in the immune system. Dendritic cells can activate helper and killer T cells, and can provide a supportive environment to develop effective B-cell responses and antibody production.
To optimize the immune response, the new study, published in Science Translational Medicine, harnessed a specific IL-12, IL-12p70. Byron Brook, Ph.D., of the Precision Vaccine Program, co-led the work with Valerie Duval, Ph.D., of the biotechnology company Combined Therapeutics, Inc.
The current BioNTech/Pfizer mRNA vaccine against COVID-19, they found, doesn't induce production of IL-12p70 in human cells. So, they designed an mRNA that explicitly directs cells to make it.
"We wanted to give the signal needed to optimize the immune response," says Dowling.
They designed the mRNA so it could stand alone, or be used as an adjuvant to turbocharge other vaccines. When they gave it to mice as an adjuvant to the BioNTech/Pfizer vaccine, the animals produced large amounts of IL-12p70 in addition to the spike protein.
The adjuvant boosted multiple elements of the immune response—not just antibody production, but also cytokine production and immune cell activity—each important for protection from SARS-CoV-2. Moreover, in aged mice, the immune response with the adjuvant reached levels similar to those in young adult mice.
The adjuvant-vaccine combination also produced more long-lasting immunity than the current vaccine alone. Animals receiving the adjuvant showed signs of amplified immunity even one year later. Though more research is needed, the adjuvant could reduce the need for frequent vaccine boosters in humans.
Reducing side effects of mRNA vaccines
The new mRNA incorporates a second technology—a so-called Multi-Organ Protection (MOP) sequence—designed to reduce side effects.
"With the MOP system you get controlled distribution just to muscle cells, where the vaccine is injected," says Dowling. "This is unconventional and a step forward."
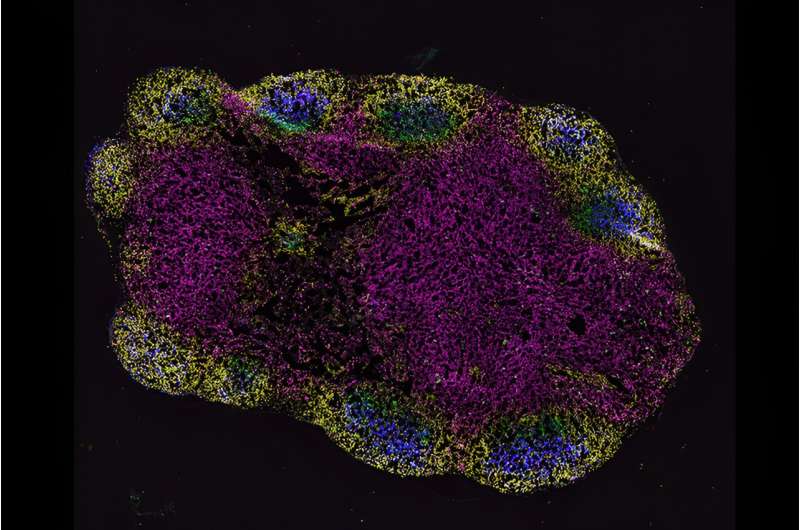
Although the mRNA does travel to cells throughout the body, the MOP sequence ensures that it acts only on muscle tissue, which the team found when they tested it in mice. In other tissue types, like vital organs, MOP binds to microRNAs inside the cells, signaling them to recycle the mRNA so they can't use it to make IL-12p70.
MOP was compatible with mRNA encoding the SARS-CoV-2 spike antigen and amplified the effects of the IL-12p70 mRNA, inducing immunity in both mice and hamsters.
Finally, because of the potency of boosting IL-12p70 production, very low doses of the BioNTech/Pfizer mRNA vaccine were needed to stimulate a strong immune response. This could help ensure there's enough vaccine supply should it be needed quickly.
"Our technology gives us the ability to reduce the vaccine dose but get the same level of immune response," says Brook. "This is what's needed for mRNA vaccines to be used more widely."
The team believes the technology could be adapted for other mRNA vaccines in development, such as flu vaccines. Alternatively, their mRNA could be given as an adjuvant together with any existing vaccine. They've moved on to testing it in primates, whose immune systems more closely resemble those of humans, with the ultimate goal of starting a Phase 1 clinical trial.
More information: Byron Brook et al, Adjuvantation of a SARS-CoV-2 mRNA vaccine with controlled tissue-specific expression of an mRNA encoding IL-12p70, Science Translational Medicine (2024). DOI: 10.1126/scitranslmed.adm8451