This article has been reviewed according to Science X's editorial process and policies. Editors have highlighted the following attributes while ensuring the content's credibility:
fact-checked
peer-reviewed publication
reputable news agency
proofread
New medications for early Alzheimer's draw praise, controversy
Independence Health System has introduced a new service that capitalizes on an overwhelming patient medical need and two new medicines.
IHS has opened Memory Clinics at its Excela Health and Butler Health System campuses, where patients are evaluated for early Alzheimer's disease and can receive infusions of the new therapies. For patients with the disease, IHS doctors administer lecanemab, and soon, donanemab, both approved by the Food & Drug Administration in the past year, but only lecanemab is now available.
Donaemab, which received FDA approval in July, is on order at IHS.
The medications have been hailed as breakthrough therapies for an incurable disease and they have also drawn controversy over their effectiveness and risks. The drugs work by attacking amyloid-beta plaques in the brain, which have been associated with Alzheimer's disease.
The medications slow, but do not stop the progression of the disease.
So far, IHS has enrolled more than 30 patients in the program, which includes testing of spinal fluid and advanced imaging techniques. The new Memory Clinics will expedite evaluation and treatment of patients with early Alzheimer's disease, said Mary Elizabeth Kovacik Eicher, director of neurosciences at IHS, who said the health system was the first in Western Pennsylvania to administer lecanemab for the disease.
Alzheimer's is a neurodegenerative disease, mostly affecting people over age 65, characterized by progressive deterioration in thinking, memory and reasoning skills. Some 50 million people worldwide are affected by the disease, which has been called a global health crisis, and has frustrated scientists who've searched for treatments for years.
But among some researchers, approval of the drugs has drawn controversy over safety and efficacy. Among the side effects of the medications, part of a class of drugs called monoclonal antibodies, were bleeding in the brain and accelerated loss of brain volume, which is also a sign of worsening Alzheimer's.
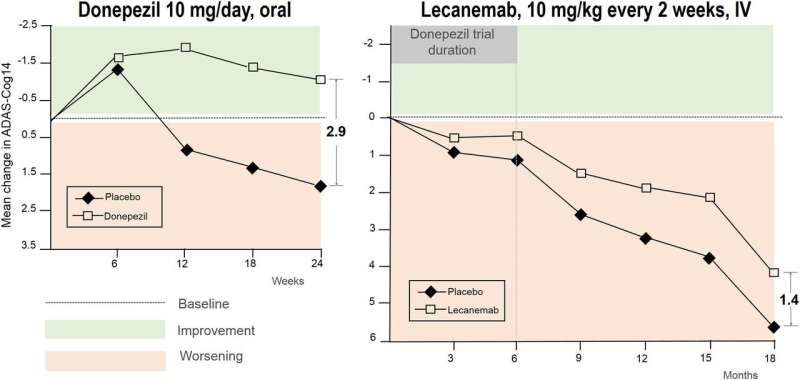
A study published in eNeuro, for example, said the drugs had a "worrisome safety profile," with 1-in-4 patients taking lecanemab experiencing brain swelling or bleeding, and more than 1-in-3 patients on donanemab experiencing the same side effects. Instead of slowing the decline of patients with the disease by 27% over 18 months, as the drug makers have said, the researchers said the reduction was no more than 2.5%.
"The small and uncertain benefits, the worrisome and poorly understood risks and the very high costs of treatment suggest that these drugs are promoted largely out of theoretical rather than practical benefits," the study concluded.
For IHS' Dr. Eicher, who is overseeing the rollout of IHS' Memory Clinics, how much the medications slow the progression of the disease isn't what matters. Medicine will always involve a cost-benefit choice, she said.
"Whether it's a 27% reduction or 2%, it's more than we've ever had before," she said. "There's a lot of controversy with these drugs because of the side effects. There is a risk here. There will always be that in medicine. There is that in everything."
The side effects her patients have experienced, such as headache, have been minimal and easily controlled, she said, but serious problems may not have shown up in such a small group.
"It depends on these patients, families and loved ones to make the decisions that are right for them," Dr. Eicher said. "Those patients deserve a chance.
"For them, it's really about hope," she said.
More information: Alberto J. Espay et al, Lecanemab and Donanemab as Therapies for Alzheimer's Disease: An Illustrated Perspective on the Data, eneuro (2024). DOI: 10.1523/ENEURO.0319-23.2024
2024 the Pittsburgh Post-Gazette. Distributed by Tribune Content Agency, LLC.