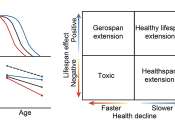
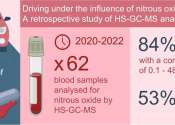
Toxic gas adds to a long history of pollution in southwest Memphis
For many years, Rose Sims had no idea what was going on inside a nondescript brick building on Florida Street a couple of miles from her modest one-story home on the southwestern side of town.
May 2, 2024
0
0