Researchers describe new molecular interactions behind the inhibition of TGF beta-signaling
(Phys.org)—Researchers headed by Maria Macias an ICREA researcher at the Institute for Research in Biomedicine (IRB Barcelona) and Joan Massagué, a Howard Hughes Medical Institute investigator at Memorial Sloan-Kettering Cancer Center (MSKCC) in New York, have identified a new molecular mechanism that plays a crucial role in the control of the activation of certain genes associated with cancer.
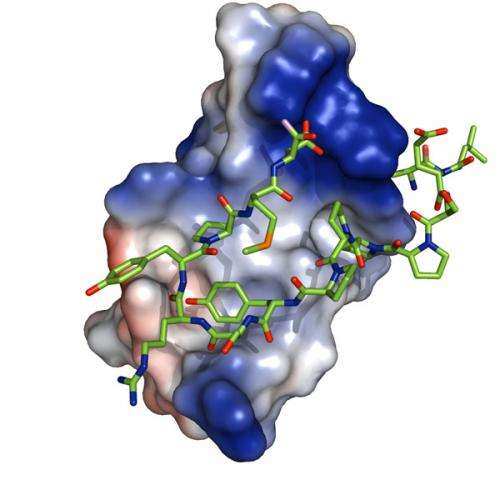
Through detailed structural and biochemical studies, the researchers identified a key domain present in a family of proteins called Smads, whose binding determines whether the transcription of genes controlled by the TGF-beta and BMP signaling cascades will be bound by activators or labeled for degradation. These processes are critical to the correct development and maintenance of tissues and organisms.
When looking at inhibitory Smads , the researchers found that the specific domain binds directly and constitutively to their targets. This is in contrast to what happens with receptor-activated Smads, where the proteins must first undergo processing by phosphorylation – a chemical change whereby the proteins are first activated and then labeled for degradation after completing their transcriptional function. The study appears online today (August 23) in the journal Structure.
Smads are key proteins in the signaling pathways of the hormones TGF-beta and BMP, which are known to participate in the control of stem cell pluripotency and differentiation and in the development and maintenance of metazoan organisms. In this study, the researchers looked at the interactions of Smad7 – a protein inhibitor of TGF-beta signaling – with molecules implicated in the cascade, including three ubiquitin ligases and YAP, a transcription coactivator. They identified the domains in the four proteins that interact with the same region of Smad7 and quantified these interactions in terms of affinity values.
Previous work by the groups on a similar type of protein, called receptor-activated Smads, has shown that in order for transcription to take place, these Smads undergo the process of phosphorylation. In this study, which focuses on inhibitory Smads, the researchers found that this step of molecular processing was not necessary and that the four proteins bind constitutively and directly to the targets.
The TGF-beta pathway is tightly regulated. Its regulation includes a feedback process whereby the two sets of Smads play complementary roles in the same signaling cascade, as they can either inhibit or trigger gene transcription, depending on cell type and the physiological needs of the tissue or organism. As with most biological processes, achieving a fine balance between the two is key, since uncontrolled gene transcription is a hallmark of serious diseases such as cancer. This latest discovery helps to shed light on how organisms achieve this balance.
One of the keys to success of this project was the unique combination of perspectives and methodologies that the partners contributed. Macias' team at IRB Barcelona used a mixture of biophysical and molecular biology techniques to decipher the minute structures of subdomains within the proteins at the atomic level. "The problem," she says, "is that we are looking at small sections of the full proteins in vitro, isolated from their cellular environment. Using techniques such as nuclear magnetic resonance, we are able to see the details down to the atoms in the binding sites. But because we zoom in so closely, we can lose sight of what the interactions we characterize can actually mean for the function of the entire protein in the cell."
Massagué's group at MSKCC was able to take each of Macias' detailed conformational changes and, using mammalian cells and full length proteins, see the effects these changes had in the cells. "Merging the detailed and bigger pictures is a difficult but key step to understanding the nature of biological processes, and to identifying what happens in disease," he says. "Detailed information on the structures of molecules involved in fundamental processes, such as that provided by this study, can tell us where to look to take to control when things go wrong."