XL-184 (Cabozantinib) goes 12-for-12 in colorectal cancer explants
The novel c-MET and VEGFR2 inhibitor, XL-184 (Cabozantinib), resulted in a significant decrease in tumor growth in 12 out of 12 colorectal cancer (CRC) patient-derived explants, with 8 of the explants exhibiting stable disease. The results of this preclinical work are presented at the AACR Annual Meeting 2013.
"With molecularly targeted agents, we typically see 3 or 4 CRC explant models with a significant decrease in tumor growth. Here we have a drug that was active in every explant we tested. It's really exciting," says John Arcaroli, PhD, investigator at the University of Colorado Cancer Center and assistant professor in the Division of Medical Oncology at the University of Colorado School of Medicine, the study's senior author. Dr. Arcaroli works with Wells Messersmith, MD, co-leader of the CU Cancer Center Developmental Therapeutics Program to develop and test novel compounds for the treatment of gastrointestinal malignancies.
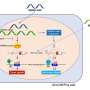
This drug appears to work by inhibiting angiogenesis – the growth of new blood vessels that tumor tissues need to supply themselves with nutrients. Unlike other drugs that target angiogenesis, XL-184 targets not only the primary driver of angiogenesis, the VEGFR2 signaling pathway, but also targets c-Met, a pathway that is important for survival of tumor cells.
"Other studies have shown that anti-VEGF therapy decreases angiogenesis, but also promotes tumor cell survival and metastasis that is dependent on the c-MET signaling pathway," Arcaroli says. "This drug inhibits both – the formation of blood vessels and a primary escape mechanism whereby tumor cells survive. This two-target approach may be the reason we're seeing very potent effects with this agent."
The group, which also includes members from the University of California, San Francisco Helen Diller Family Comprehensive Cancer Center and from Harvard Medical School, is now investigating the mechanisms by which the compound reduces tumor growth. The group also plans additional explant testing in order to identify predictive biomarkers of sensitivity or resistance to XL-184. In combination, this work will likely lead to a Phase II biomarker-driven clinical trial of the drug in patients with metastatic colorectal cancer.