New safety test predicts reactions to novel drugs and cosmetics
A simple lab-based skin test which eliminates the risk of adverse reactions to new drugs, cosmetics and household chemicals has been developed by a Newcastle University, UK team.
It uses real human skin and immune cells to show any reaction such as a rash or blistering indicating a wider immune response within the body.
The development is timely as it offers a reliable alternative for the cosmetic industry as a ban on the sale of any cosmetic product tested on animals came into effect across Europe in March.
Professor Anne Dickinson from the Institute of Cellular Medicine recently presented the technology at the In-Vitro Testing Industrial Platform (IVTIP) conference in Brussels. She said: "This skin assay offers an accurate and rapid alternative to animal testing and provides the bridge between the laboratory tests for novel drugs and the first stage of clinical trials in humans.
"It is accurate and faster than anything currently around and can save companies time and resources. The test identifies drugs or products which are likely to cause a reaction or just not work effectively in humans."
The test called Skimune, which is trademarked and has a patent pending, has been successfully tested by a number of large pharmaceutical companies on drugs in development and provides a reliable result within two weeks.
By revealing skin sensitisation or an adverse reaction that may not be identified by use of an animal or computer model, the assay can provide vital information which will allow a drug company to make informed decisions earlier saving significant development costs.
Professor Dickinson said: "We've already shown this works as a way of testing new drugs for adverse immune reactions that can't be identified when tested in animal models."
Working with the National Institute of Biological Standards and Control (NIBSC) the Newcastle team have been testing monoclonal antibodies for adverse responses. Professor Dickinson added: "Our Skimune test would have predicted the terrible outcome at Northwick Park in 2006. Then six men taking part in a clinical trial had severe reactions to a monoclonal antibody resulting in organ failure. Previous laboratory and animal research gave no indication that this was likely to occur.
"Our test would have picked up the risk because it is a skin-based model of the human immune response."
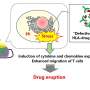
The skin assay has been developed using cells isolated from blood samples from a range of healthy volunteers. Differentiated into dendritic cells which activate the T-cells, these in turn create a cytokine storm. Useful for fighting infection, if this immune response goes unchecked it can be extremely harmful to the individual. Skimune provides a histology skin damage read out enabling the severity and potency of reaction to be gauged.
Professor Richard Stebbings, principle scientist at NIBSC welcomed the development adding: "This assay offers a valuable alternative to animal models, used for safety testing of biological medicines and which are often poorly predictive of human responses."
Professor Anne Dickinson has spent 20 years working to understand how we prevent the body rejecting donor tissue such as bone marrow. This technology has been developed from a skin explant model for predicting a potentially serious complication of bone marrow transplantation, 'graft versus host' disease - a common complication following the transplant.
It has been supported by the UK's innovation agency with a Technology Strategy Board grant for the development of a prototype.
As well as patent pending the Skimune test, the Newcastle University team have set up a company Alcyomics Limited which aims to take the technology forward to offer personalised medicine, enabling an individual to be tested for drug responses.
More information: More information on the technology can be found on www.alcyomics.com