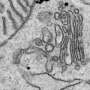
High-resolution 3-D imaging draws new picture of Golgi's whereabouts during cell division
Resolving a fundamental question in cell biology and showing off the powers of new high-resolution 3-D imaging, NIH scientists have discovered where the Golgi apparatus, which sorts newly synthesized proteins for transport inside and outside the cell, goes when it disassembles during cell division, according to research to be presented on Sunday, Dec. 15, at the American Association for Cell Biology (ASCB) annual meeting in New Orleans.
With conventional microscopy techniques, the scientists said they could only watch as the Golgi dissolved into tiny "puncta" and an unresolvable haze. But powerful new imaging techniques allowed the researchers to follow the Golgi through its "choreographed disassembly process," which now appears tightly linked to the endoplasmic reticulum (ER) during cell division, said Dylan Burnette, Ph.D., and Prabuddha Sengupta, Ph.D., and Jennifer Lippincott-Schwartz, Ph.D., of the Eunice Shriver National Institute of Child Health and Human Development (NICHD) in Bethesda, MD.
Cell division by mitosis is the complicated yet critical process by which a mother cell divides into two daughter cells. But first, the mother cell has to pack up her cellular household contents, disassembling and dividing up everything for her soon-to-be-formed daughters.
How cells manage division has been exhaustively studied for over a century and yet basic mysteries remained. Scientists knew that some organelles such as the ER are pulled apart before division but keep their tubular membrane structure intact. Other organelles such as the Golgi, go to pieces after the prophase of mitosis through choreographed disassembly.
But where does the Golgi go once it is in pieces? To answer the question, the NIH researchers started with two plausible theories: In the endoplasmic reticulum (ER)-linked hypothesis, the Golgi puncta and enzyme haze are closely held by the ER; in the non-ER-linked model, the puncta and haze float about on their own, waiting for cytokinesis when the two daughter cells separate and the Golgi body reappears as stacks of membrane-bound cisternae, ready to sort proteins from the reassembled ER.
Powered by their new imaging technologies, which gave them far greater resolution than previously possible, the researchers saw clear support of the ER-linked model—the enzyme haze sticking close to ER markers with the puncta clustering near ER exits.
For a second line of proof, the NICHD researchers followed up with a pharmacological-based trapping assay that showed Golgi enzymes being held tightly by the ER during mitosis. The results indicate that Golgi enzymes redistribute into the ER during mitosis, and that they must follow an ER export pathway to reform the Golgi at the end of mitosis.
This study not only resolves a basic cellular question but shows what new solutions await as these new technologies give us keener vision and sharper tools.
More information: Author will present, "High-resolution imaging of Golgi protein trafficking through the ER during mitosis," on Sunday, Dec. 15, in the 12 noon to 1:30 p.m. poster session, "Establishing and Maintaining Organelle Structure."