Aging brains need 'chaperone' proteins

(Medical Xpress)—The word "chaperone" refers to an adult who keeps teenagers from acting up at a dance or overnight trip. It also describes a type of protein that can guard the brain against its own troublemakers: misfolded proteins that are involved in several neurodegenerative diseases.
Researchers at Emory University School of Medicine have demonstrated that as animals age, their brains are more vulnerable to misfolded proteins, partly because of a decline in chaperone activity.
The researchers were studying a model of spinocerebellar ataxia, but the findings have implications for understanding other diseases, such as Alzheimer's, Parkinson's and Huntington's. They also identified targets for potential therapies: bolstering levels of either a particular chaperone or a growth factor in brain cells can protect against the toxic effects of misfolded proteins.
The results were published this week in the journal Neuron.
Scientists led by Shihua Li, MD, and Xiao-Jiang Li, MD, PhD devised a system in which production of a misfolding-prone protein that causes a form of spinocerebellar ataxia can be triggered artificially in mice at various ages. Both Li's are professors of human genetics at Emory University School of Medicine. The first author of the paper is BCDB graduate student Su Yang.
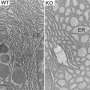
Spinocerebellar ataxia is an inherited neurodegenerative disease in which patients develop gait problems and a loss of coordination in mid-life, because of atrophy of the cerebellum. There are several types, each caused by a mutation in a different gene.
Most of the mutations that cause spinocerebellar ataxia involve an expansion of a "polyglutamine repeat" in a protein. Having the same protein building block (the amino acid glutamine) repeated dozens of times alters the protein's function and makes it more likely to misfold and clump together. The misfolded proteins are toxic and interfere with the normal forms of the same protein.
Huntington's disease is caused by a similar polyglutamine repeat. Misfolded proteins also play roles in Alzheimer's and Parkinson's, although their production is not driven by an inherited polyglutamine repeat in those diseases.
Li's team was trying to distinguish between two possibilities. One was that the duration of mutant protein accumulation is important for disease severity; aging might allow more misfolded proteins to accumulate and become toxic over time.
Instead, the scientists observed that older animals develop disease more quickly after mutant protein production is triggered. The mutant protein accumulates more quickly in 9- and 14-month old mice than in 3-month old mice, suggesting that aged neurons are more vulnerable to the effects of the misfolded protein.
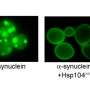
Chaperones are proteins whose job is to "prevent improper liaisons" between other proteins; they prevent the sticky regions of proteins from grabbing something they're not supposed to. Li's team identified a particular chaperone called Hsc70 whose activity declines with age in the brain, while others' activity does not.
To confirm Hsc70's importance, the researchers showed that boosting cells' levels of Hsc70 can bolster their ability to cope with misfolded proteins. Injecting mice in the cerebellum with a virus that forces cells to make more Hsc70 can slow degeneration. The researchers found that the mutant protein interferes with production of a growth factor called MANF (mesenchephalic astrocyte-derived neurotrophic factor) in the cerebellum and that Hsc70 can prevent this interference. Injection of a virus that forces cells to make more MANF can also slow degeneration.
Potentially, small molecules that increase Hsc70 or MANF levels could be used for treating spinocerebellar ataxia, says Xiao-Jiang Li.
More information: S. Yang, S. Huang, M.A. Gaertig, X.J. Li and S. Li Age-dependent decrease in chaperone activity impairs MANF expression leading to Purkinje cell degeneration in inducible SCA17 mice. Neuron (2013).