New RNA interference technique finds seven genes for head and neck cancer
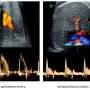
(Medical Xpress)—In the hunt for genetic mutations that cause cancer, there is a lot of white noise. So although genetic sequencing has identified hundreds of genetic alterations linked to tumors, it's still an enormous challenge to figure out which ones are actually responsible for the growth and metastasis of cancer. Scientists in Rockefeller's Laboratory of Mammalian Cell Biology and Development have created a new technique that can weed out that noise—eliminating the random bystander genes and identifying the ones that are critical for cancer. Applying their technique to head and neck cancers, they've discovered seven new tumor-suppressor genes whose role in cancer was previously unknown.
The new technique, which the lab recently applied to a screen for skin tumor genes, is particularly useful because it takes a fraction of the resources and much less time than the traditional method for determining gene function—breeding genetically modified animals to study the impact of missing genes.
"Using knockout mice, which are model organisms bred to have a particular gene missing, is not feasible when there are 800 potential head and neck cancer genes to sort through," says Daniel Schramek, a postdoctoral fellow in the lab, which is headed by Rebecca C. Lancefield Professor Elaine Fuchs. "It can take about two years per gene. Our method can assess about 300 genes in a single mouse, in as little as five weeks."
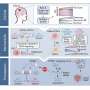
The researchers made use of RNA interference, a natural process whereby RNA molecules inhibit gene expression. They took short pieces of RNA which are able to turn off the function of specific genes, attached them to highly concentrated viruses, and then, using ultrasound to guide the needle without damaging surrounding tissue, they injected the viruses into the sacs of mouse embryos.
"The virus is absorbed and integrated into the chromosomes of the single layer of surface cells that cover the tiny embryo," explains Fuchs. "As the embryo develops, this layer of cells becomes the skin, mammary glands and oral tissue, enabling us to efficiently, selectively and quickly eliminate the expression of any desired gene in these tissues. The non-invasive method avoids triggering a wound or inflammatory response that is typically associated with conventional methods to knockdown a gene in cultured cells and then engraft the cells onto a mouse."
When the mice grew, the researchers determined which genes, when turned off, were promoting tumor growth, and what they found was surprising.
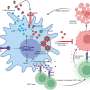
"Among the seven novel tumor suppressor genes we found, our strongest hit was Myh9, which codes for the protein myosin IIa, a motor protein with well-known function in cell structure and cell migration," says Schramek. "Through further functional studies we found that myosin IIa is also required for activation of the main guardian of the genome—a tumor suppressor protein called p53."
The lab showed that when the myosin IIa gene was mutated, p53 was not able to build up in the cell nucleus, and chaos ensued: genes responsible for repairing damaged cells and killing off tumor cells were not activated, and invasive carcinomas spread within three months.
The researchers devised a strategy to reactivate p53 in these cells, and showed in vitro that tumor suppression was restored. "Head and neck cancers are the sixth most deadly type of cancer worldwide. Interestingly, Myh9 is also mutated in human head and neck cancers, and low expression of myosin IIa correlates with poor prognosis for the patient," says Fuchs. The group hopes to examine the effect in clinical trials in the future, and plans to look at the function of the other six genes their study identified.
"We've demonstrated that this method of RNA interference is highly useful in the rapid discovery, validation and characterization of tumor suppressor genes that might otherwise be missed in a genetic screen," says Schramek. "It can be applied to many kinds of cancers, such as breast and lung."
More information: "Direct in vivo RNAi screen unveils myosin IIa as a tumor suppressor of squamous cell carcinomas." Daniel Schramek, Ataman Sendoel, Jeremy P. Segal, Slobodan Beronja, Evan Heller, Daniel Oristian, Boris Reva and Elaine Fuchs Science 343: 309–313 (January 17, 2014) DOI: 10.1126/science.1248627