How developing sperm stick to the right path
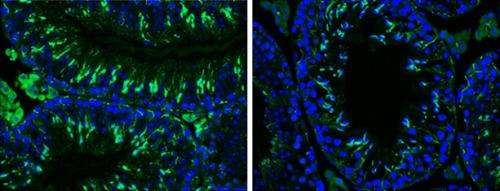
The process of producing high-quality, fertile sperm requires many steps. A study in The Journal of Cell Biology shows how the transcription factor p73 promotes this process by regulating the adhesions between developing sperm and their support cells.
The p53 family of transcription factors has an ancient and well-conserved function in protecting reproductive cells. In mammals, for example, p63 promotes the death of eggs and sperm that have sustained DNA damage, and female mice lacking p73 are infertile due to defects in egg development. Male mice lacking p73 are also infertile, but the reason for this is unknown. Led by Ute Moll, a team of researchers from the University of Göttingen in Germany and Stony Brook University in New York studied the testes of p73-deficient rodents to find out why.
Sperm develop in the multilayered epithelia of seminiferous tubules in the testes. This process is enabled and organized by Sertoli cells, which span the seminiferous epithelium and tightly envelop maturing sperm cells at each stage of their development in "nursing pouches," guiding their differentiation and movement toward the surface, where they release mature, motile sperm.
The researchers found that developing sperm cells lacking p73 detached from the epithelium prematurely and died. The p73-deficient cells showed altered expression levels of many proteins that regulate adhesions between the developing sperm and their Sertoli nurse cells. Sertoli cells don't express p73, but they were also affected by the loss of adhesion in p73-deficient testes, losing their characteristic morphology as well as the inter-Sertoli cell adhesions that form the blood–testis barrier, which protects developing sperm from circulating immune cells and toxins.
The results demonstrate how p73 ensures male fertility in mice by regulating critical cell adhesions that enable sperm maturation. Moll now wants to investigate whether mutations in p73 also can cause human infertility.
More information: Holembowski, L., et al. 2014. J. Cell Biol. DOI: 10.1083/jcb.201306066
















