May 13, 2014 feature
Staying focused: Cortico-thalamic pathway filters relevant sensory cues from perceptual input
(Medical Xpress)—On the one hand, the nervous has limited computational capability – but at the same time, the sensory environment contains an immense amount of information. In this demanding situation, the brain somehow manages to selectively focus attention on relevant stimuli. Recently, scientists at Technische Universität München, Munich and Ruhr University Bochum, Bochum investigated thalamic tactile sensory relay by employing optogenetics (the use of light to control neurons which have been genetically sensitized to light) to control specific cortical input to the thalamus. They show that the deepest cortical layer (known as layer six, or simply L6) plays a key role in controlling thalamic signal transformation (specifically, by controlling adaptive responses of thalamic neurons) and thalamic gating of dynamic sensory input patterns by changing the firing mode.
Dr. Rebecca A. Mease and Dr. Alexander Groh discussed the paper they and Prof. Patrik Krieger published in Proceedings of the National Academy of Sciences. In this study they investigated how the brain actively controls and gates information reaching higher stages of cortical processing by using optogenetics to turn on specific cortical input to the thalamus and measure how this impacts the processing of sensory signals in the thalamus.
"Sensory signaling is a millisecond-timescale process that involves an as-of-yet unknown number of neuronal circuits," Groh tells Medical Xpress. "Understanding a complex brain function – in this case, the relay and filtering of sensory information – requires the dissection of neuronal circuits in quasi-real time. Moreover, sensory processing can only be studied in the intact, working brain in living animals. " This is technically challenging, he notes, because it is difficult to identify different circuit components and isolate their impact – but optogenetics, cell type-specific targeting of functional probes in combination with deep brain whole-cell recordings have now made it possible to study specific neuronal networks and their function in higher brain processes, in which the thalamo-cortical network is known to play an essential role.
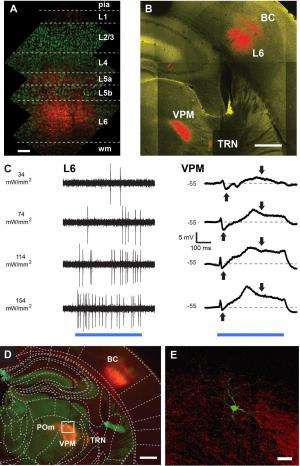
"We know now that the thalamo-cortical network is not a simple one-way route that only relays sensory information to the cortex," Groh continues. "Instead, deep layers of the cortex send information back to the thalamus via massive axonal projections." In fact, he illustrates, one of the two cortico-thalamic pathways originates in L6, and provides one of the major inputs to thalamic nuclei. "Compared to the other cortical layers, L6 has been least investigated, partially because of its inaccessibility and cell type diversity," Groh explains. "Using cell-type-specific stimulation allowed us to specifically activate L6 and study its effect on thalamic sensory processing."
With these tools, the researchers showed that L6 impacts these thalamic signaling properties by controlling the resting membrane potential and the availability of transient low-threshold (T-type) calcium channels – that is, low-voltage-activated calcium channels that open during membrane depolarization. The deep brain structure in vivo patch clamp technique they used to address these questions was another key procedure. "Patch clamp – a technique developed1,2 by our mentor Prof. Bert Sakmann, in collaboration with Erwin Neher – is a standard technique in brain slices, but is still difficult to establish in the working brain – especially in its deep structures," Mease tells Medical Xpress. (Sakmann and Neher were jointly awarded the 1991 Nobel Prize in Physiology or Medicine for their work concerning the patch clamp as well the function of single ion channels in cells.) "It was only with this approach that it was possible for us to record the relay of sensory information by single thalamic neurons and determine how cortical feedback from layer 6 impacts this process." In this case, she adds, extracellular recording of neural spikes would not have exposed how L6 activation changes the size and adaptation of excitatory postsynaptic potentials (EPSPs) – temporary depolarizations of postsynaptic membrane potential caused by the flow of positively charged ions into the postsynaptic cell as a result of opening of ion channels.
While Groh says that the scientists did not develop any new essential techniques in this paper in order to address these questions, he points out that their discovery was made possible by combining state-of-the-art techniques including optogenetics, a new transgenic mouse known as nstr1, and whole-cell recordings of single neurons in deep brain structures. "For this project, we needed both optogenetics and cell-specific targeting of L6 neurons using this particular mouse line," Groh explains, adding that "the transgenic mouse line was very kindly given to us by Nathaniel Heintz and Eric Schmidt from Rockefeller University. Earlier methods to manipulate neuronal networks often used electrical stimulation, which does not discriminate between cell types and the spatial extent is not well controlled – so the combination of cell type-specific activation with light overcomes these limitations, and allowed us to specifically study the impact of L6 on thalamic relay modes." In particular, the role of cortical inputs in thalamic processing has been difficult to address using electrical stimulation, because electrical activation of the cortex also activates thalamic neurons via their axonal endings in the cortex – an unwanted side effect that would make the interpretation of the data very difficult.
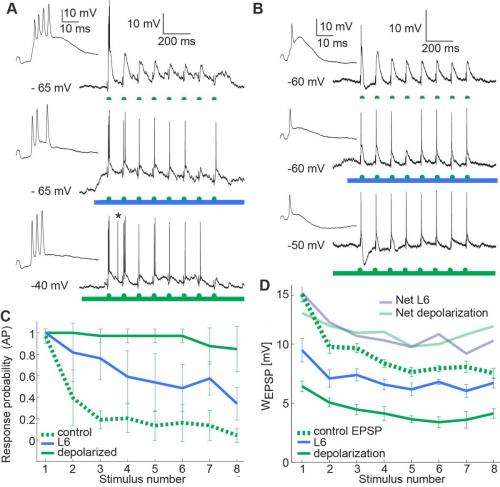
In so doing, the researchers found that by inducing depolarization, this corticothalamic pathway effectively regulates how thalamic neurons respond to sensory signals. Activity in the L6 pathway switches the thalamus into tonic mode, one of two basic physiological states (the other being burst mode) in which each thalamic projection neuron can exist: In tonic mode, the neurons translate sensory input into single action potentials; in burst mode (also known as oscillatory mode) neurons respond with clusters of high frequency spikes. "By activating this pathway optogenetically," Mease says, "we showed that if L6 is active, an invariant sensory stimulus can evoke two different patterns of activity in the thalamus. In addition, we extended these findings by testing if the same pathway serves to optimize the relay of sensory details. We found that information about the stimulus – in this case frequency – is more reliably relayed when the cortical input to the thalamus is activated." She points out that this is achieved by a decrease in thalamic adaptation – a well-known feature of sensory physiology.
Mease also commented on the finding that while both tonic and burst modes have been described during anesthesia/sleep and wakefulness/behavior, there is a pronounced shift toward the tonic mode during alertness. "Excellent work by others in the field has shown that the binary breakdown between burst and tonic modes is something of a false dichotomy, and that thalamic spiking is more of a continuum between these extremes," Mease explains. "L6 activation does increase the tendency for tonic firing, so it could be that L6 serves as an attentional signal, but this possibility needs to be tested in awake animals."
"Our measurements support the long-standing hypothesis, elegantly formulated by Murray Sherman from the University of Chicago, that the burst-associated inattentive thalamus is optimized to detect sudden changes, such as the occurrence of a new stimulus, and convert these into a strong signal to the cortex as a rapid burst of spikes,"3 says Groh – "a process Prof. Sherman termed 'a wake-up call for the cortex'4 in a review of a paper by Swadlow and Gusev5. Subsequently, cortical activity via L6 can switch the operational mode of recipient thalamic neurons to tonic coding."
The researchers extended these findings, notes Groh, by addressing if L6 feedback affects the temporal relay of sensory frequency information through the thalamus. "For sensory physiologists, this is of interest, since frequency information is an important cue in sensory signals. Frequency information in visual, auditory and somatosensory signals is processed by sensory systems because it proved useful for the brain to handle this information. To study frequency coding in the thalamocortical system we presented repetitive whisker inputs to the mice as done many times before in other labs. However, when we activated the L6 pathway in these experiments, responses of thalamic neurons reflected these repetitions more accurately, demonstrating that frequency information is more effectively transmitted through the thalamus when L6 is active."
The cortico-thalamic pathway is conserved across most mammalian sensory system, meaning that rodents, cats, primates and humans show a common architecture of two feedback pathways from cortex to thalamus in the auditory, visual and somatosensory (but not olfactory) systems. "While this was not our finding, it is a general property of corticothalamic systems," Mease notes, "It is possible that L6 input to thalamus might serve a similar role in sensory gating in other modes of sensation."
"Incoming sensory information is constantly changing," she continues, "and not all of this information is salient to the animal, because most may be redundant or irrelevant to the behavioral task at hand." Mease points out that the nervous system handles this by employing adaptive mechanisms at many different physical and temporal scales, from synaptic depression within milliseconds, to inactivation of ion channels over minutes. This means that for a given stimulus, the output neural response will not be static, but will depend on recent stimulus and response history. This adaptive behavior is a fundamental property of neural processing, and allows neurons to better use their dynamic range of output responses to encode a wide range of stimulus properties. "We find that L6 input serves to dampen frequency-dependent adaptation, so subsequently more stimuli reach the cortex."
That being said, there are broader implications of L6 input to the thalamus shaping both the overall gain and the temporal dynamics of sensory responses that reach the cortex. "Evolutionary advantage is created by versatility – specifically, the capability to master different conditions when inhabiting different areas or niches," Groh says. "This versatility comes with the price that more information is sampled than is needed in a particular situation." As a result, the brain has evolved mechanisms to attend to the important details in a particular situation while ignoring uninteresting signals (which, Groh says, arguably represent the majority of sensory input). He concludes that their finding suggests that the cortex can finely tune its own input through gain control (dampening or boosting overall thalamic signals) and filtering (controlling temporal resolution of patterns).
When asked if, given that (1) sensory signals en route to the cortex undergo profound signal transformations in the thalamus, (2) a key thalamic transformation is sensory adaptation in which neural output adjusts to the statistics and dynamics of past stimuli, and (3) the thalamus, hypothalamus and hippocampus being part of the limbic system, might memory reconsolidation play a role in the cortico-thalamic pathway? "It's conceivable that the cortico-thalamic pathway is subject to long term plasticity," Groh conjectures. "In fact, on a synaptic level, these inputs can change their strength and retain the adjusted strength for long periods. This process represents another albeit much slower form of adaptation which some interpret as memory.
Conversely, might the thalamic-cortical pathway affect memory? "If particular sensory-evoked activity patterns would cause long-term changes in the cortico-thalamic pathway, and thereby change the way incoming signals are processed before reaching the cortex," he opines, "then this would indeed reflect a form of information storage."
Moving forward, Groh says that there are several projects, planned or in process, that build on the results of their paper. One such project addresses the time-varying effect of L6 activation – that is, how are sensory signals relayed when L6 is only briefly activated (as opposed to during ongoing elevated activity? "The idea is that the early hyperpolarizing effect that we did not investigate here imposes a threshold for sensory input to switch the processing mode to high fidelity processing, but also ensures high detectability of new stimuli by priming individual neurons. It may be that the relative timing of hyperpolarization and depolarization helps to determine which stimuli are encoded in detail," Groh explains.
"One of the main questions we will pursue," he continues, "is how this pathway operates in the waking brain – in particular, how it affects behavior, especially given that many other groups have reported a mix of burst and tonic spiking in awake animals." For example, he illustrates, under which circumstances is it advantageous to relay sensory signals in burst mode? Relatedly, he adds, they will also look at which behavioral tasks are better solved when the cortico-thalamic pathway is active. "In the end, we hope to better understand why nature developed the switch at all. This may then even enable us to understand which pathological conditions are caused by dysfunctions of cortico-thalamic feedback.
"Moreover," Groh continues, "Understanding what determines the balance between the hyperpolarization and depolarization that L6 evokes in thalamic neurons is an issue that needs to be further explored in the intact brain." Other groups have found that this variability is due to the alignment of two areas of the mouse brain: the cortical barrel, an area in the somatosensory cortex representing input from the contralateral side of the body; and the thalamic barreloid, a set of structures in the ventral posterior medial nucleus of the thalamus, each associated with a particular whisker. "This variability is most likely functionally relevant, and therefore should be addressed in a more systematic manner," he stresses. "The hyperpolarizing effect of this pathway that we and others have described is probably also relevant to how thalamic neurons process sensory stimuli, but this needs to be studied during sensory processing. For this study," he continues, "we focused on the main stable effect of L6, which was a reset of the thalamic membrane potential during the period of increased L6 activity."
Groh notes that this stable depolarization can be regarded as the result of a longer – that is, greater than 200 ms – activation of this pathway, which may occur when an object is examined more closely and/or when motor centers are required for examination, such as during whisker movements. "The time dependence of cortical L6 feedback is indeed very interesting, and we haven't touched upon this mechanism in the paper. That said, however, it's likely that the outcome of cortical feedback on thalamic sensory processing can vary significantly, depending on the duration of layer 6 activation."
In addition, Groh points out that there are other innovations they consider applying in this context. "Moving to the behavioral level requires technical development," he says. "Fortunately the individual techniques have been developed by other researchers and colleagues – but a combined approach needs to be established. It's also of great interest to study the commonalities of thalamocortical pathways in different modalities. In this study we looked at the processing of touch information, and we'd like to know how the homologous pathways affect visual or auditory processing. It's fascinating that despite the fundamental differences between visual, auditory and somatosensory signals, the basic layouts of the thalamocortical systems for each modality are quite similar."
In closing, Groh tells Phys.org that other areas of research that might benefit from their study include "any medical research involving the thalamo-cortical system – for example, epilepsy, which is caused by pathological brain oscillations – or other conditions that might involve inappropriate gating of sensory signals, such as chronic pain, schizophrenia, or panic disorders. However, independently of immediate practical consequences, in the long run it's important to understand the basics of neuronal systems."
More information: Cortical control of adaptation and sensory relay mode in the thalamus, Proceedings of the National Academy of Sciences, Published online before print on April 18, 2014, doi:10.1073/pnas.1318665111
Related:
1The extracellular patch clamp: a method for resolving currents through individual open channels in biological membranes, Pflugers Archiv 1978 Jul 18;375(2):219-28 (PDF)
2Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches, Pflugers Archiv August 1981, Volume 391, Issue 2, pp 85-100, doi:10.1007/BF00656997
3Burst and tonic firing in thalamic cells of unanesthetized, behaving monkeys, Visual Neuroscience (2000) 17:55-62 (PDF)
4A wake-up call from the thalamus, Nature Neuroscience 4, 344 - 346 (2001), doi:10.1038/85973 (PDF)
5The impact of 'bursting' thalamic impulses at a neocortical synapse, Nature Neuroscience 4, 402 - 408 (2001), doi:10.1038/86054
© 2014 Medical Xpress