Mechanism discovered for attaching an 'on' switch that helps cells accessorize proteins
St. Jude Children's Research Hospital scientists have discovered how an important "on" switch is attached to the machinery that cells rely on to adapt thousands of proteins to meet changing conditions. The research appears in the current issue of the journal Cell.
The switch is a small protein called NEDD8. Problems with NEDD8 have been associated with several cancers, developmental disorders and infectivity of the human immunodeficiency virus (HIV), which causes AIDS. Drugs that target NEDD8 are in anti-cancer clinical trials. The ability of HIV to evade the anti-viral immune response depends in part on the ability of the virus to hijack the NEDD8 machinery.
NEDD8 is also a key component of the machinery that cells use to adapt to changing conditions. Just as individuals adapt to changes in their environment by donning gloves, boots, hats and other accessories, cells adapt by "accessorizing" proteins to modify their function.
NEDD8 is a specialized accessory. It functions as the "on" switch for accessorizing 10 to 20 percent of the thousands of proteins that do the work of cells. Those accessories mark some proteins for elimination, others for a change in function and others for relocation to different parts of the cell. Until now, however, how NEDD8 slipped into position was unknown.
Researchers showed how part of the machinery for accessorizing proteins, a component called cullin-RING, is first modified by NEDD8. The addition of NEDD8 transforms the ability of cullin-RING to accessorize other proteins. Those proteins are involved in important biological functions such as cell division, immune response and embryonic development.
"This discovery is a major advance in understanding the machinery cells use to regulate an astonishingly vast number of proteins they depend on as well as the diseases that arise when the system malfunctions," said corresponding author Brenda Schulman, Ph.D., a member of the St. Jude Department of Structural Biology and a Howard Hughes Medical Institute (HHMI) investigator.
Schulman and her colleagues study the machinery that manages the accessorizing process, whether the accessory is NEDD8 or a different small protein called ubiquitin. Ubiquitin accessorizes proteins though a process known as ubiquitination. Cullin-RING, which NEDD8 accessorizes, is a major command center of ubiquitination.
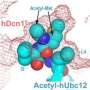
This study builds on an observation first author Daniel Scott, Ph.D., made shortly after joining Schulman's laboratory in 2006. Scott, an HHMI research specialist III, showed that while ubiquitin could be coaxed into binding to and accessorizing cullin-RING, NEDD8 was the preferred partner.
Scott used a technique called X-ray crystallography to capture a crystal structure that explained why. In the process, investigators determined for the first time that different components of the ubiquitination machinery work cooperatively to align NEDD8 and cullin-RING. That alignment promotes the transfer of NEDD8 rather than ubiquitin to the proper site on cullin-RING. The transfer of NEDD8 allows other proteins to be accessorized with ubiquitin.
The mechanism outlined in this research establishes a paradigm for understanding protein regulation in cells, Schulman said. "This research sets the stage for broadly understanding this key aspect of protein regulation in cells," Scott said.