New technology reveals insights into mechanisms underlying amyloid diseases
Amyloid diseases, such as Alzheimer's disease, type 2 diabetes, cataracts, and the spongiform encephalopathies, all share the common trait that proteins aggregate into long fibers which then form plaques. Yet in vitro studies have found that neither the amylin monomer precursors nor the plaques themselves are very toxic. New evidence using two-dimensional infrared (2D IR) spectroscopy has revealed an intermediate structure during the amylin aggregation pathway that may explain toxicity, opening a window for possible interventions, according to a report in the current issue of Biomedical Spectroscopy and Imaging.
"Figuring out how and why amyloid plaques form is exceedingly difficult, because one needs to follow the atomic shapes of the protein molecules as they assemble. Most tools in biology give either shapes or motions, but not both. We have been developing a new spectroscopic tool, called two-dimensional infrared spectroscopy, which can monitor the plaques as they form in a test tube," said lead investigator Martin T. Zanni, PhD, of the Department of Chemistry at the University of Wisconsin-Madison.
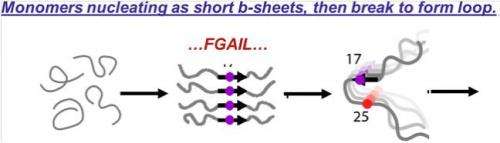
The investigators employed this new technology to study the amyloid protein associated with type 2 diabetes. Isotope labeling was used to measure the secondary structure content of individual residues. By following many 2D IR spectra from one particular region (known as the FGAIL region) over several hours, they were able to visualize the amylin as it progressed from monomers to fibers.
"We learned that, prior to making the plaques, the proteins first assemble into an unexpected and intriguing intermediate and organized structure," commented Dr. Zanni. The proteins undergo a transition from disordered coil (in the monomer), to ordered β-sheet (in the oligomer) to disordered structure again (in the fiber).
These results help to elucidate the physics of the aggregation process, the chemistry of amyloid inhibitors, and the biology of type 2 diabetes, as well as clarify previously contradictory data.
The authors suggest that differences between species in their capacity to develop type 2 diabetes may be related to the capacity to form these intermediate amylin structures. That may be why humans develop the disease while dogs and rats do not. "I am not encouraging us to begin engineering our DNA to match that of rats, but our findings may help to develop plaque inhibitors or hormone replacement therapies for people suffering from type 2 diabetes, because we know the structure we want to avoid," says Dr. Zanni. He adds that mutations in the FGAIL region may inhibit fiber formation by interfering with the formation of these intermediates.
More information: "Insights into amylin aggregation by 2D IR spectroscopy," by Tianqi O. Zhang, Lauren E. Buchanan, Martin T. Zanni. Biomedical Spectroscopy and Imaging, Volume 3/Issue 3. DOI: 10.3233/BSI-140078