Researchers find clue to stopping Alzheimer's-like diseases
(Medical Xpress)—Tiny differences in mice that make them peculiarly resistant to a family of conditions that includes Alzheimer's, Parkinson's and Creutzfeldt-Jakob Disease may provide clues for treatments in humans.
Amyloid diseases are often incurable because drug designers cannot identify the events that cause them to start.
Professor Sheena Radford, Astbury Professor of Biophysics at the University of Leeds, said: "Amyloid diseases are associated with the build-up of fibrous plaques out of long strings of 'misfolding' proteins, but it is not clear what kicks the process off. That means the normal approach of designing a drug to destroy or disable the species that start the disease process does not work.
"We have to take a completely different tack: instead of targeting the cause of the disease, we need to disrupt the plaque building process."
The University of Leeds-led team's study, published in the journal Molecular Cell today, looked to mice for a way forward.
"We already knew that mice were not prone to the build up of some of these plaques. This study, for the first time, observed the building happening and saw the differences between the mice proteins and their almost identical human equivalents," Professor Radford said.
She added: "We mixed the mice and human proteins and found that the mice protein actually stopped the formation of the plaque-forming fibrils by the human protein."
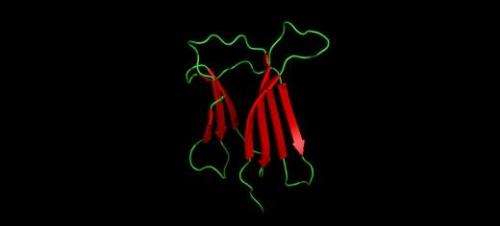
The research was conducted completely in the test-tube using human and mice beta-2 microglobulin proteins produced in the laboratory. Plaques made up of beta-2 microglobulin are associated with Dialysis Related Amyloidosis (DRA). Instead of being a neurodegenerative condition like Alzheimer's or Parkinson's, DRA primarily affects the joints of people on kidney dialysis.
The team observed differences in the formation of the plaque-forming fibrils in samples containing only mice protein, samples with only the human protein and samples containing mixtures of the two.
The lead researcher, Dr Theodoros Karamanos, said: "These two versions of the proteins are almost exactly the same, with very slight differences in structure, but the outcomes are completely different. If I put a misfolding-prone protein in the human sample, I see the formation of fibrils in two days in the right conditions. If I do the same in the mouse sample, I can leave it for weeks and there are no fibrils.
Dr Karamanos added: "The exciting thing is that if you mix the proteins—with only one mouse protein for every five human proteins—you see a significant disruption of the formation of fibrils."
The study used Nuclear Magnetic Resonance spectroscopy to look at a molecular level at the interactions of the different proteins and identified tiny differences in the physical and chemical properties of the surfaces that made a great difference to whether plaques are formed.
The results showed that the mouse protein binds to the human protein more tightly than the human protein binds to its misfolded form. Interestingly, subtle differences in the driving forces of binding (i.e. the balance of hydrophobic and charge-charge interactions) in the binding interface govern the outcome of assembly.
Dr Karamanos said: "We can't just load up a syringe and inject mouse protein into patients. But if we know the properties of the interface between the two proteins that are responsible for the inhibition effect, we can ask the chemists to design small molecule drugs which mimic what the mouse protein does to the human protein. That may be a key insight into how to stop the plaque building process."
More information: Theodoros K. Karamanos et. al., 'Visualization of Transient Protein-Protein Interactions that Promote or Inhibit Amyloid Assembly,' Molecular Cell (2014) DOI: 10.1016/j.molcel.2014.05.026 ; URL: dx.doi.org/10.1016/j.molcel.2014.05.026