August 19, 2014 report
Better living through mitochondrial derived vesicles
(Medical Xpress)—As principal transformers of bacteria, organelles, synapses, and cells, vesicles might be said to be the stuff of life. One need look no further than the rapid rise to prominence of The international Society for Extracellular Vesicles, or its prestigious journal, for confirmation of their broad power. While cell biologists now have a good handle on the gross observables of vesicles—their size, composition, and contents—little is understood about their social character. In other words if you are a cellular-scale entity and someone sends you a mysterious 50nm spherical package, should you open it, ignore it, or fuse it with a detoxifying peroxisome as fast as you can?
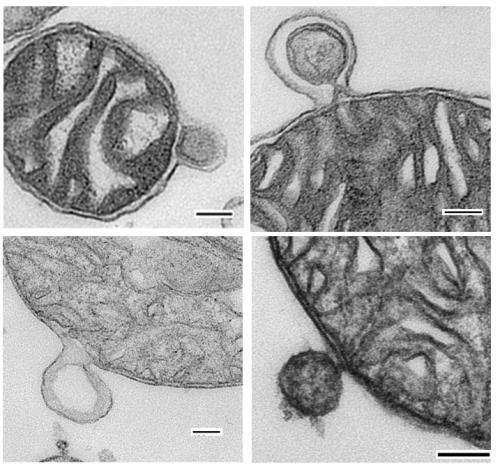
Perhaps the best way explore this conundrum is to take a look at the vesicular mannerisms of those most sociable of creatures, our mitochondrial endosymbionts. As quick to fuse with you as they are to autodestruct or even split in two, these organelles also have ample provisions to generate small garden variety vesicles, appropriately known as mitochondrial-derived vesicles (MDVs). Indeed this ability is believed to have been preserved intact across evolutionary timescales from when the ancestors of mitochondria were feral bacteria. A remarkable exploration into the world of MDVs was recently published in the EMBO journal by corresponding author Heidi McBride and her colleagues. Their review describes different ways these vesicles can be effluxed from a complex double-membraned organelle (and potentially, abosorbed), and considers their fate once at large within the cell.
Although MDVs were originally associated with the transport of outer membrane protein to peroxisomes, other MDVs were soon found consorting with late endosomes or multivesicular bodies. Multivesicular bodies can fuse with the plasma membrane of the cell and subsequently release vesicles themselves called exosomes. The recent explosion in the discovery of diverse functions of exosomes in different places has led to a veritable bestiary of terms for them, each mundane to its own corner of life. For example, dendritic cells release dexosomes, cancer cells release oncosomes, and prostate cancer cells specifically release prostasomes.
A similar exuberance parallels the naming for the various intracellular compartments where vesicles are processed and degraded. Vesicles therefore can have intracellular fusion partners anywhere on the endo-auto-lyso-phagosome spectrum. For events where it's not just a vesicle but rather whole mitochondria that forms the secretory cargo, we have the more esoteric terms of transexudation and transmitophagy. The theme that unites all these "somes" is quality control. Mitochondrial survival is not an all-or-nothing decision but rather a continuum that slides from the activities of simple proteases and ubiquin-mediated handling of oxidized or unfolded proteins, to bulk elimination of proteins via MDVs, to full-blown mitophagy.
One should not conclude, however, that mitophagy or complete mitochondrial apoptosis would beget a "mitosome". Mitosomes, while derived from mitochondria, are a different ballgame entirely. Their main job, at least within the creatures that have them, is to generate iron sulfur clusters and ship them to the ends of the cell. Their main claims to fame are that they have completely lost their mtDNA, their capacity to respire, and most of their import machinery.
Normal mitochondria, as opposed to mitosomes, can contain upwards of 1000 different proteins in addition to several varieties of RNA from various sources. Efforts to analyze the contents and potential fusion machinery of isolated vesicles using mass spectroscopy will help to identify their diverse functions. A quick trip over to "Vesiclepedia" indicates that up to 10% of secreted proteins (determined from proteomic analysis) are mitochondrial. The emerging picture is that the potential domain of a single mitochondria, and its influence, can no longer be viewed as just its own host cell. Long held suspicions of interspecies transfer of mitochondrial genomes in plants or amphibians become more credible as the mechnisms of transfer become better understood.
Finding the highlights of any comprehensive review is certainly a subjective affair. Among the juicier anecdotes found in the paper may be the primary manifestations of a condition known as Zellweger syndrome. Here mutations in core peroxisomal import proteins cause a lack of peroxisomes and cell death through accumulation of very long-chain fatty acids and ROS production. The main symptom of interest, at least for the brain, is a deficit in myelination.
The brain is the place where vesicle processing has been perfected into an artform. Everyone "knows" neurons talk to their own synapses using electrical signals, but when the time comes to knock on the door of a neighbor they invariable turn to vesicles to do their bidding. Vesicles can't be all nasty or nobody would take them or their contents in. As many likely know, glutamate (one of the go to synaptic vesicle transmitters) may be toxic at high concentration but it is also a highly coveted primary metabolic precursor. A few of the more directly irritating transmitters on the other hand, will sometimes pepper their stock with tasty peptides.
The habit of packaging wastes, or even outright assaults, with gifts is an old one, and the difference between toxin and antidote sometimes a small one. The complex cembranoids responsible for the strong aroma of tobacco plants, for example, can directy counteract the effects of nicotine at receptor sites. The taming of the vesicle through the city life of multicellularity has led to many new innovations. Understanding their role in mitochondria will help understand their larger roles in the affairs of the organism.
More information: A new pathway for mitochondrial quality control: mitochondrial-derived vesicles, The EMBO Journal, onlinelibrary.wiley.com/doi/10 … j.201488104/abstract
Abstract
The last decade has been marked by tremendous progress in our understanding of the cell biology of mitochondria, with the identification of molecules and mechanisms that regulate their fusion, fission, motility, and the architectural transitions within the inner membrane. More importantly, the manipulation of these machineries in tissues has provided links between mitochondrial dynamics and physiology. Indeed, just as the proteins required for fusion and fission were identified, they were quickly linked to both rare and common human diseases. This highlighted the critical importance of this emerging field to medicine, with new hopes of finding drugable targets for numerous pathologies, from neurodegenerative diseases to inflammation and cancer. In the midst of these exciting new discoveries, an unexpected new aspect of mitochondrial cell biology has been uncovered; the generation of small vesicular carriers that transport mitochondrial proteins and lipids to other intracellular organelles. These mitochondrial-derived vesicles (MDVs) were first found to transport a mitochondrial outer membrane protein MAPL to a subpopulation of peroxisomes. However, other MDVs did not target peroxisomes and instead fused with the late endosome, or multivesicular body. The Parkinson's disease-associated proteins Vps35, Parkin, and PINK1 are involved in the biogenesis of a subset of these MDVs, linking this novel trafficking pathway to human disease. In this review, we outline what has been learned about the mechanisms and functional importance of MDV transport and speculate on the greater impact of these pathways in cellular physiology.
© 2014 Medical Xpress