June 18, 2014 report
Control of secretory trafficking in neurons
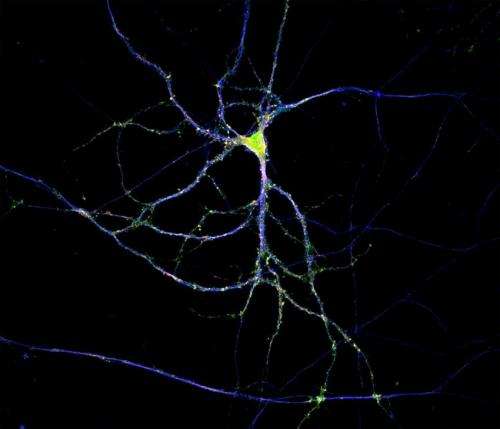
(Medical Xpress)—Neuroscience typically proceeds by trimming back previous overly dogmatic statements. Such is the case with new findings that proteins are locally manufactured to order throughout an extended neuronal tree. While it was once thought that only the cell body and dendrites could finesse the proteins that need to be placed in the membrane (or beyond into the extracellular space), it is now known that such "secretory trafficking" takes place both there and everywhere—apparently even in parts unknown at the tips of axons.
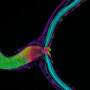
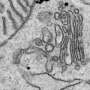
The mechanisms that control the transit of membrane and secreted proteins through neurite-based endoplasmic compartment (ER), and subsequently the trans-Golgi network, are largely unknown. Ultrastructural evidence from classic studies shows "nissl bodies" in synapses, but limitations in the older dye methods have let to erroneous assumptions about their ubiquity. More modern imaging has revealed "golgi outposts" at axon branch points, but gives little information about their dynamics. A paper just published the journal Cell Reports now shows that synaptic activity is a major regulator of the whole works. This extends the theme of spike-based control of major housekeeping functions, like myelination and mitochondrial transport, down into local synaptic control of all major neural construction projects.
Cytoplasmic proteins, diffusable enzymes and skeleton components are essential to the neuron, but the the real franchise players—the proteins that perform all those intangibles required for neurons to act as a team—are the membrane-based receptors and ion channels. Using live-cell imaging and fluorescent reporters of transport through the secretory pathway, the researchers could track packages of proteins as they emerged from the ER. The trick to make this possible was to activate a temperature-sensitive viral mutant known as VSVG-ts045. At 40°C, the fluorescently tagged VSVG is synthesized and accumulated in the ER but unable to exit. Switching the temperature to 20°C causes the VSVG to released to next stage of processing.
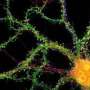
The researchers found that these so-called "tubulovesicular carriers" underwent long-range transport throughout the dendrite when synaptic activity was inhibited. When they boosted synaptic activity pharmacologically, these post-ER carriers were spatially confined and accumulated at the plasma membrane. They further found that this activity-dependent confinement requires Ca2+/calmodulin-dependent protein kinase (CaMK) activity and phosphorylation of KIF17. KIF17 is a kinesin motor which is known to transport NMDA receptor proteins along the plus-end direction of microtubules in dendrites. That may sound like a pretty narrow function for KIF, but in actuality it is likely a much more general protein involved in transport of many components, and perhaps mRNAs.
The authors suggest that what we have here, namely the activity-dependent control of microtubule-based transport, is a mechanism for tuning the length of neural trees. Other researchers have found evidence for secretory protein synthesis and trafficking in axons. Axons are a whole new ballgame by virtue of the their unique cytoskeleton and the long distances they cover. Intriguing new research indicates yet another breach of contract neuroscience: mitochondria are transported down axons and into other cells. In the optic nerve head near the retina, for example, they are drawn into axonal protrusions that are coerced into existence by neighboring astrocytes. In a process originally called transexudation, but now refined as transmitophagy, the bulk of the local mitochondria are taken up by astrocytes and assimilated.
These behaviors are not just chance observations but rather essential behaviors. In the case just described the researchers found that an even greater proportion of retinal ganglion cell mitochondria are degraded at the optic nerve head then in the ganglion cell soma which they might call home. This kind of auto-phagocytic absorption with lysosomal fusion happens elsewhere in the eye, namely in the turnover of photoreceptor outer segments assisted by retinal pigment epithelial cells. At an even more primal level, strikingly similar mechanisms are responsible for degradation of sperm mitochondria once the egg is penetrated.
Closely regulated transfer of secreted proteins and mRNAs not just within the brain, but to and from the brain could scarely have been imagined before, at least to the degree we now see it. Vesicle-mediated transfer of genetic information between the hematopoietic system and the brain, for example, is one recent case in point. We are now firmly entrenched in a new and more powerful neuroscience. A science which seeks to explain the integrated function of the cell where structure and measurable activity become one and the same, and extend beyond the borders of the cell.
More information: Paper: Synaptic Control of Secretory Trafficking in Dendrites, Cell Reports, www.cell.com/cell-reports/full … 2211-1247(14)00420-3
© 2014 Medical Xpress