Pre-clinical model reveals pathology behind mitochondrial disease
A lack of a particular mitochondrial protein in genetically manipulated mice has shown to trigger events in the body that causes mild heart hypertrophy (enlargement) and severe fatty liver disease.
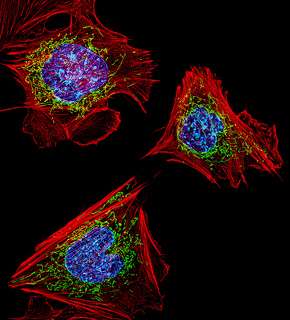
Mitochondria are organelles found in every cell of the body. They have their own set of genes and are responsible for energy production.
Mitochondrial disease is complex, most often caused by a defect in mitochondrial protein formation which impairs the energy generating machinery and makes patients suffer from a shortage of energy.
Harry Perkins Institute of Medical Research research associate Dr Tara Richman says: "the biggest gap in our knowledge with mitochondrial disease is how regulation of gene expression of mitochondrial proteins happens."
Together with her UWA colleagues she is closer to understanding how proteins encoded by DNA in the cell nucleus need to cooperate with proteins encoded from mitochondrial DNA to effectively produce energy in mitochondria.
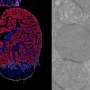
Focussing on what happens in a tailor-made mutant mouse model almost devoid of the mitochondrial protein MRPS34, she found that physiological changes were most pronounced in tissues 'hungry for energy', like the heart and liver.
The researchers anticipated the brain would also show detrimental signs of MRPS34 deficiency, but this tissue appeared unharmed with no change in behaviour.
MRPS34 is the first mitochondrial protein studied in a live mouse model for mitochondrial disease, which permits insight into the pathological mechanism and disease development over time.
Characterising human disease in mice
"We created a mouse model with a mutation that caused the mice to have reduced levels of the MRPS34 protein," Dr Richman says.
The researchers studied the fully developed mutant mice (phenotype) and worked backwards to discover how a shortage of this protein (genotype) influenced animal growth and development.
Due to less MRPS34 in mitochondria, it appeared there was not enough mitochondrial complexes required to sustain proper energy metabolism.
"We knew from this that MRPS34 was important in mitochondrial function but we didn't know why" she says.
They now understand that MRPS34 is important for the formation and stabilisation of the mitochondrial ribosomes, which in turn are essential for the building of new mitochondrial proteins like MRPS34.
"The downstream effect is dysfunction of the mitochondria overall."
The researchers do not know why the mutation, present in every cell of the mouse body, did not lead to damage in more tissues.
Dr Richman hopes further studies with this mouse model will explain why the mutation-driven outcomes increased in severity with age and how one can intervene.
More information: "Mutation in MRPS34 Compromises Protein Synthesis and Causes Mitochondrial Dysfunction." PLoS Genet 11(3): e1005089. DOI: 10.1371/journal.pgen.1005089