Role of breast cell infection in flu transmission between mothers and breast-feeding ferrets
Influenza is known as an infectious respiratory disease, but a study published on October 8th in PLOS Pathogens suggests that infected cells in breast tissues could play a role in virus transmission from mothers to breast-feeding infants and vice versa using a ferret model.
Alyson Kelvin, from the University Health Network, Toronto, Canada, and colleagues developed a novel infant-mother ferret influenza model utilizing nursing mother ferrets and their 4-week-old infants to investigate the host-pathogen interactions of influenza virus infection between mothers and breast-feeding infants. Ferrets are known to be infected by human flu viruses, and can transmit the virus from ferret-to-ferret in a fashion that is believed to mimic the human situation.
Initially, the researchers infected either mothers or infants through nasal inoculation with the 2009 H1N1 strain of influenza virus and closely followed the health of both animals. When the infants were exposed, both they and—a few days later—their mothers developed influenza with symptoms in both upper and lower respiratory tracts. Similarly, when mothers were exposed to the virus, first they and subsequently their nursing young became ill with influenza, again involving upper and lower respiratory tracts.
As mammary glands are a significant point of contact between mothers and infants, the researchers then investigated the susceptibility of cells in mammary glands to influenza infection and the breast tissue as a possible source of virus transmission. They examined virus presence within mammary tissue as well as virus in the mother's milk.
All ferret mothers tested had at least one mammary gland that contained infectious influenza virus. Live influenza virus was present in the nipples of positive mammary glands, and was also found in the milk of mothers of inoculated infants. This shows that mammary glands are able to harbor live influenza virus, and that live virus can be shed into the milk of secondarily infected mothers.
When the researchers directly inoculated the ferret mammary gland, they found that its cells could be directly infected by influenza virus and then were able to produce live virus. Infants nursed by these mothers became infected themselves, likely through the breast because the transmitted virus was found in the infants before it was detectable in nasal liquid from the mothers. However, transmission through direct contact of infected mothers or infected infants through a non-breast feeding route cannot be ruled out. Just like virus transmitted from the mother's respiratory tract, transmission that appeared to occur through the mammary gland led to severe disease and mortality in the ferret infants.
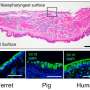
Although the researchers then showed that isolated human mammary cells can also be directly infected by the flu virus, this by no means proves that human breast tissue becomes infected during influenza infection. Comparing gene expression patterns in infected ferret mammary cells with uninfected ones, they saw substantial changes that suggest that besides eliciting an immune response, influenza infection of mammary gland cells shuts off milk production genes and activates genes involved in cell proliferation and growth.
Looking at whole mammary glands from ferrets, the researchers also saw changes indicating the cessation of milk production, but it still remains to be determined whether the changes seen in cultured human cells have any effects on milk production or on breast tissue in flu-infected people. For instance, it is well documented that ferrets are more susceptible to influenza H5N1 virus systemic influenza virus infection than humans, and therefore it is still possible that spreading of the virus to mammary cells is a ferret-specific event not seen in humans. In any case, the present study emphasizes that we know little about influenza transmission, and that more studies are needed in humans and in animal models to understand how the virus moves from host to host.
Referring to data that Middle-Eastern Respiratory Syndrome (MERS) virus might be transmitted to humans from camel milk, the researchers say, together with their findings "support a hypothesis that respiratory viruses may have the ability to also infect mammary tissue, including human breast cells, possibly due to a shared branched architecture and cellular structure of epithelial cells in lungs and mammary glands".
Regarding the public health implications, they emphasize that their data "reinforce the importance of seasonal influenza vaccination in pregnant and breastfeeding women". Because current guidelines focus on respiratory transmission, they also suggest that "further investigation and guideline development for the management of influenza transmission between mothers and infants may be important."
More information: Paquette SG, Banner D, Huang SSH, Almansa R, Leon A, Xu L, et al. (2015) Influenza Transmission in the Mother-Infant Dyad Leads to Severe Disease, Mammary Gland Infection, and Pathogenesis by Regulating Host Responses. PLoS Pathog 11(10): e1005173. DOI: 10.1371/journal.ppat.1005173