Molecular switch may sensitize triple-negative breast cancers to immunotherapy
Previous studies at the University of Colorado Cancer Center show that the experimental drug AMPI-109 potently kills triple-negative breast cancer cells. But even the most compelling evidence of cell death in a dish isn't enough to push a drug into human clinical trials, even for triple-negative breast cancer, which has a high mortality rate and remains largely without targeted treatment options. Clinical trials are commonly guided by the knowledge of how a drug works - an understanding that can allow researchers to tweak a drug's effectiveness or explore rational combinations of multiple drugs to maximize antitumor responses. Now a study published in the journal Oncogenesis offers compelling evidence that AMPI-109 works by flipping the switch on an enzyme called PRL-3 that initially puts cancer cells to "sleep" or senescence, and shortly thereafter leads to their death, or apoptosis.
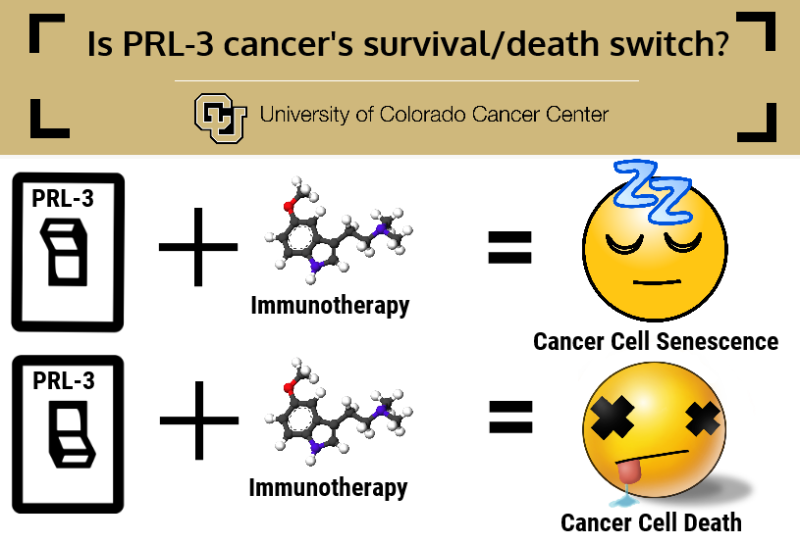
"For decades, we've known about a paradoxical signaling pathway called TNF-R1 whose activation can either help a cell survive or lead to cell death. However, the signals that lead to this pathway promoting survival or promoting death have been poorly understood, especially in the context of cancer cells. We have observed that one regulator of this process in triple-negative breast cancer cells may be the activity of PRL-3. With this gene active, cells survive. With PRL-3 inactivated, cells senesce and eventually die," says Hamid Gari, PhD, who studied the mechanism of PRL-3 while working as a doctoral candidate in the lab of CU Cancer Center investigator James R. Lambert, PhD. Gari is first author and Lambert is senior author of the current study which was performed in collaboration with Scott Lucia, MD in the department of Pathology.
Gari explains that PRL-3 sets in motion a set of genes that recruits elements of the immune system to boost cancer growth during good times and allows cancer cells to sleep through bad times, for example those caused by anti-cancer therapies.
"Hamid's studies knocked down the gene PRL-3 in triple-negative breast cancer cells using genetic techniques, but the drug does something analogous by blocking PRL-3 function. Our studies suggest AMPI-109 reprograms the cell to enter senescence but then they keep going past this state and into apoptosis," Lambert says.
The finding comes at a time when cancer immunotherapies are becoming first-line treatments for many forms of the disease. Basically, the strategy is to teach the immune system to recognize and attack tumor tissue. However, some cancers may be particularly good at "hiding" from the immune system, allowing them to subsist and thrive in challenging tumor tissue conditions. For this reason, many immunotherapies result in holding cancer at bay rather than wiping it out completely. In fact, some immunotherapies treat cancer as a chronic condition, with therapy continuing indefinitely with the goal of simply keeping cancer in check.
"Our studies propose that by inhibiting PRL-3 activity, such as with AMPI-109, it may serve as a 'flag' to signal the immune system where the tumor is, and in essence could sensitize tumors to immunotherapy. The result is a two-hit strategy to expose the tumor and then allow the immune system combat it," Gari says.
The group has applied for NIH funding and is also working toward company funding for studies of the drug's safety. If results remain promising, AMPI-109 could become an important tool in an oncologist's kit to target triple-negative breast cancer with immunotherapy.
More information: H H Gari et al, Loss of the oncogenic phosphatase PRL-3 promotes a TNF-R1 feedback loop that mediates triple-negative breast cancer growth, Oncogenesis (2016). DOI: 10.1038/oncsis.2016.50