Overabundance of common species in 'microbiome' may cause inflammation
A new study led by UC San Francisco scientists shows that a bacterium commonly found in the human gut is overrepresented in patients with a rare, often disabling autoimmune disease known as neuromyelitis optica, or NMO. The researchers suggest that the greater population of this bacterium, Clostridium perfringens, that they observed in NMO patients may play a role in development of the disorder.
In NMO, which until quite recently was thought to be an unusual form of multiple scerlosis (MS), the immune system attacks neural support cells called astrocytes. Specifically, NMO targets one type of the astrocytes' water channels, structures also known as aquaporins, which allow water to rapidly flow in and out of cells. Repeated immune assaults on these channels in the spinal cord and optic nerve create lesions that can lead to paralysis or blindness. Unlike MS, there are no approved therapies for NMO.
"We don't know what causes NMO, but we wondered if some organism could be fooling the immune system into attacking itself," said UCSF's Scott Zamvil, MD, PhD, senior author of the study, published online in the August 4, 2016 issue of Annals of Neurology. (An early, unedited version of the study was previously published online by the journal on July 11, 2016.)
While cautioning that the study was small, involving only 16 NMO patients, Zamvil, professor of neurology, said the work provides support for a theory of "collateral damage" that he and others are actively exploring: a certain amino acid sequence of a C. perfringens protein matches a sequence in the aquaporin targets in NMO, which may prompt the immune system to launch an attack on the bacterium that also incidentally targets the water channels.
Zamvil and colleagues compared stool samples from the NMO patients to samples from 16 multiple sclerosis (MS) patients, and to those from 16 healthy individuals without either disease. Patients with MS were included in the comparison because both NMO and MS patients are treated with a drug that can potentially alter the composition of the microbiome, the term for the collections of microbes resident in and on the body.
NMO patients' gut microbiomes were found to contain significantly more C. perfringens, compared to either MS patients or healthy participants. Though there were also differences among the three groups in the representation of 41 other bacterial groups, no single species was as significantly over-enriched as C. perfringens among patients with NMO.
"Based on the microbiomes of the three study groups, NMO patients look even less like healthy controls than MS patients do," Zamvil said, even though some NMO and MS patients had received the same drug treatment.
Despite the small size of the study, it still took many years to put together. The rarity of NMO presented one challenge, but a second was a matter of decorum. "Patients come in and get blood drawn all the time – no problem. But if you meet a patient for the first time, it's not the most diplomatic thing to ask for a stool sample," Zamvil said.
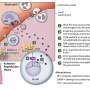
Zamvil had previously discovered a 90 percent similarity between a sequence of ten amino acids in the aquaporin targeted in NMO, known as aquaporin 4, and in C. perfringens. (The discovery of aquaporins earned the Nobel Prize in Chemistry in 2003 for Peter Agre, MD, of The Johns Hopkins University.)
This "molecular mimicry" is commonly seen in autoimmune diseases because antibodies designed to fight a pathogen can also target matching structures in the body itself. For example, Streptococcus ("strep") infection can prime the immune system to later attack the body, causing rheumatic heart disease or the neurological disorder Sydenham chorea.
Zamvil and colleagues had also previously found that C. perfringens itself encourages T cells in the gut to develop into pro-inflammatory immune cells called Th17 cells, which have been linked to other disorders such as rheumatoid arthritis and MS.
Because C. perfringens is a so-called "commensal bacterium" that is also found in the guts of healthy people, molecular mimicry cannot be the sole cause of NMO. Still, the bacterium's dual role as both a molecular mimic and promoter of Th17 cells suggest it could be involved in the onset of disease. "We think overrepresentation of the bug itself may make the environment pro-inflammatory, which is one step towards the development of NMO," Zamvil said.
To establish whether a cause-and-effect relationship exists between C. perfringens and NMO, Zamvil plans to perform fecal transplants in mice, feeding them the microbiome-containing fecal material of NMO patients to see if this increases occurrence of the disorder in the mice. Additional molecular experiments could also determine whether the water channel-attacking antibody in NMO is also reactive to C. perfringens.
"These preliminary results don't mean that we're promoting antibiotics to kill C. perfringens, and it also doesn't mean we're promoting over-the-counter probiotics," Zamvil cautioned, "but they do suggest that manipulation of the microbiome could be beneficial in NMO treatment in the future."