Study raises new concerns about radiation and breast cancer
It is well established that exposure to ionizing radiation can result in mutations or other genetic damage that cause cells to turn cancerous. Now a new study led by researchers with the U.S. Department of Energy's Lawrence Berkeley National Laboratory (Berkeley Lab) has revealed another way in which radiation can promote cancer development. Working with cultures of human breast cells, the researchers discovered that radiation exposure can alter the environment surrounding the cells so that future cells are more likely to become cancerous.
"Our work shows that radiation can change the microenvironment of breast cells, and this in turn can allow the growth of abnormal cells with a long-lived phenotype that has a much greater potential to be cancerous," says Paul Yaswen, a cell biologist and breast cancer research specialist with Berkeley Lab's Life Sciences Division.
A cell's phenotype is its full complement of observable physical or biochemical characteristics. Different cells can have phenotypes that look dramatically different or exhibit radically different behavior even though their genetic makeup (genotype) is identical. Signals from outside the cell can alter a cell's phenotype by regulating (or de-regulating) the cell's use of its genes. Studies have shown that if a cell develops a pre-cancerous phenotype, it can pass on these "epigenetic" changes to its daughters, just as it can pass on genetic mutations.
"Many in the cancer research community, especially radiobiologists, have been slow to acknowledge and incorporate in their work the idea that cells in human tissues are not independent entities, but are highly communicative with each other and with their microenvironment," Yaswen says. "We provide new evidence that potential cancer agents and their effects must be evaluated at a systems level."
Yaswen is the corresponding author of a paper describing this study that appears in the on-line journal Breast Cancer Research, titled "Promotion of variant human mammary epithelial cell outgrowth by ionizing radiation: an agent-based model supported by in vitro studies." Co-authoring the paper were Rituparna Mukhopadhyay, Sylvain Costes, Alexey Bazarov, William Hines and Mary Helen Barcellos-Hoff.
"The work we did was performed with non-lethal but fairly substantial doses of radiation, unlike what a woman would be exposed to during a routine mammogram," says Yaswen, who is also a member of the Bay Area Breast Cancer and the Environment Research Center. "However, the levels of radiation involved in other procedures, such as CT scans or radiotherapy, do start to approach the levels used in our experiments and could represent sources of concern."
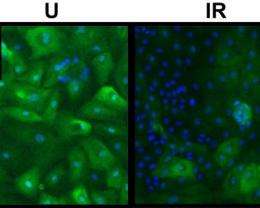
For their study, Yaswen and his collaborators worked with human mammary epithelial cells (HMECs), the cells that line breast ducts, where most breast cancers begin. In a culture dish, the vast majority of breast cells display a phenotype that allows them to divide between five and 20 times before becoming senescent. However, also present are rare variant HMECs, which display a phenotype that allows them to continue dividing for many weeks in culture. This vHMEC phenotype arises spontaneously and is much more susceptible to malignancy because it lacks a tumor-suppressing protein called p16.
To test the effects of radiation on cellular environment and subsequent cell behavior, the research team grew sets of HMECs from normal breast tissue in culture dishes for about a week, then exposed each set to a single treatment of a low-to-moderate dose of radiation. They then compared the irradiated sets to sets of breast cells that were not irradiated. Four to six weeks after the radiation treatments, most of the cells in both the irradiated and unirradiated sets had permanently stopped dividing.
"However, the daughters of breast cells exposed to radiation formed larger, more numerous patches of cells with the vHMEC phenotype than did the daughters of the unirradiated cells," Yaswen says. "An agent-based model developed by Sylvain Costes and Mary Helen Barcellos-Hoff suggests that the radiation increased the rate at which short-lived cells became senescent."
In a culture dish, breast cells will only divide and grow so long as there is room for daughter cells to spread out. When the dish becomes full, the cells stop dividing. By promoting premature senescence in the normal HMEC, the radiation treatments accelerated the outgrowth of the vHMECs.
"Radiation exposure did not directly induce new vHMECs and the effect was not dose-dependent in the dose range we investigated," Yaswen says. "However, by getting normal cells to prematurely age and stop dividing, the radiation exposure created space for epigenetically altered cells that would otherwise have been filled by normal cells. In other words, the radiation promoted the growth of pre-cancerous cells by making the environment that surrounded the cells more hospitable to their continued growth."















