Potential treatment for Chikungunya discovered
The Singapore Immunology Network (SIgN), an institute of the Agency of Science, Technology and Research (A*STAR), and VIVALIS, a French biopharmaceutical company, announced today the discovery of two new fully human monoclonal antibodies which could battle Chikungunya, a disease that currently has no available vaccine or specific treatment. The international team of scientists, coordinated by Dr Lucile Warter of SIgN, has published their groundbreaking discovery in the Journal of Immunology.
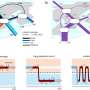
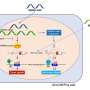
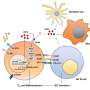
Chikungunya is prevalent in Africa, South Asia, and South-East Asia and is transmitted by the Aedes mosquito, the same mosquito that spreads dengue fever. In Singapore alone, over 1000 Chikungunya cases were reported over the period 2008-2010. Dr Warter and her collaborators used Humalex®, a VIVALIS technology platform designed to identify and generate fully human monoclonal antibodies, to develop two antibodies that could neutralize several Chikungunya strains in vitro by culturing immune cells from an individual who had developed resistance to Chikungunya. Monoclonal antibodies can be more potent and have fewer side effects than conventional small molecule drugs.
"The discovery of these antibodies is a big step forward in combating a disease that presently has no available vaccine or specific treatment. The use of VIVALIS' Humalex was invaluable in helping us isolate the target antibodies from the cultured immune cells. We hope to further validate the use of these antibodies as a viable treatment for Chikungunya." said Dr Warter. She added that further testing in vivo would have to be carried out to validate the antibodies' performance as a potential treatment for Chikungunya.
"It is thanks to the successful synergy between industry and SIgN that the development of two antibodies against a disease that is on the rise has been accomplished. The combination of Humalex technology, SIgN's expertise in human immunology, virology and molecular biology, and Singapore's location as a hub for Asia helped to speed up the selection, sequencing and characterization of the most potent antibody candidates. I am delighted to note that this breakthrough was achieved in less than a year from the start of the project", said SIgN Chairman Prof Philippe Kourilsky.
"The new platform used by SIgN for the generation of fully human monoclonal antibody is already providing excellent results, and we hope to generate a number of new fully human monoclonal antibodies that could be used as therapeutics," said SIgN Scientific Director Prof. Paola Castagnoli.
"The discovery of these new fully human monoclonal antibodies with strong neutralizing activities against the Chikungunya virus constitutes an an additional testimony of the efficiency of the Humalex platform. Coming shortly after the signature of a major commercial agreement with sanofi Pasteur, the vaccine division of Sanofi Aventis, this discovery is a further validation of the power of VIVALIS Humalex® antibody discovery platform. It is a first milestone in our research partnership with SIgN and we expect to benefit from the excellent scientific input from SIgN scientists in the field of immunology in future collaborative programs", commented Franck Grimaud, CEO, and Majid Mehtali, CSO, co-managers of VIVALIS.
More information: "Chikungunya Virus Envelope-Specific Human Monoclonal Antibodies with Broad Neutralization Potency", Journal of Immunology.