Key mutations act cooperatively to fuel aggressive brain tumor
Mutations in three pathways important for suppressing tumors cooperate to launch glioblastoma, an aggressive brain tumor that strikes children and adults. But new research from St. Jude Children's Research Hospital scientists shows those changes alone are not sufficient to cause cancer. Tumor formation requires additional mutations, some affecting different points in the same disrupted regulatory pathways.
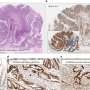
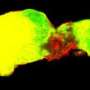
Researchers demonstrated that in mouse models of glioblastoma, tumors develop in several regions of the brain.
The findings, as well as the technique investigators used to generate them, are now being used as a possible tool for understanding patients' responses to investigational therapies that target some of the same pathways. The research appears in the March 15 edition of the scientific journal Cancer Cell.
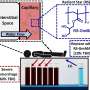
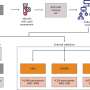
The work builds on previous studies that linked glioblastoma to disruptions in the RB1, p53 and Pten pathways, each of which has a key role in preventing tumor formation. For this study, St. Jude investigators developed a novel system that allowed them to delete the genes, either singly or in various combinations, for which the pathways are named. Researchers then tracked the impact of those deletions on the brains of adult mice.
Gliomas are the most common primary malignant brain tumor, representing more than half of the 18,000 malignant brain tumors diagnosed annually in the U.S. These tumors remain the second most common cause of cancer death among individuals ages 15 through 44. Survival is less than 10 percent for patients with the most aggressive gliomas subtype, glioblastoma.
"In this study, we set out to look at the contribution of each of these pathways and how they cooperate to generate gliomas," said Suzanne Baker, Ph.D., a member of the St. Jude Department of Developmental Neurobiology and the paper's senior author. "When you analyze a human tumor, you see all of the accumulated mutations and you don't really know in what order they happened. This experimental system provides an opportunity to initiate a tumor with specific mutations and then ask: What else gives the tumor a selective advantage?"
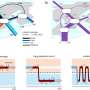
Investigators found that when Pten, Tp53 and RB1 were all silenced, mice quickly developed high-grade gliomas. In contrast, losing just one of the genes failed to generate the tumors at a high frequency.
In this experimental model, loss of Tp53 was essential for tumor formation, suggesting the gene plays an important role in launching the disease. "But other mutations are still necessary," Baker said.
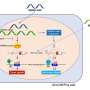
An analysis of DNA deletions and duplications in the tumors that developed in mice suggests the initiating mutation strongly influences which additional mutations provide a selective advantage to the rogue cell. For example, researchers found genes in the PI3 kinase pathway were commonly amplified in tumors sparked by Pten deletion, but were less common if Rb deletion was also introduced at the initiation of the experiment. Other genes in the Rb pathway were also amplified in tumors regardless of whether Rb deletion was one of the initiating mutations. These pathways regulate cell differentiation, proliferation and death.
The study also addresses the ongoing question of where gliomas begin. Previous work pointed to the brain niche where neural stem cells are found as the origin. Neural stem cells are the undifferentiated cells that give rise to the other, more specialized components of the nervous system. But in this study, 22 percent of the 63 gliomas developed in regions of the brain and spinal cord far from such niches. "That is consistent with what happens in humans and shows that the tumors can certainly develop in areas of the brain outside of the proliferative niches where stem cells are found," Baker said.
The cooperation and complexity identified in this study as driving tumor development have Baker and her colleagues asking new questions. Some focus on a family of drugs known as kinase inhibitors that target some of the same pathways this study showed work cooperatively to drive cancer. "Our mouse model gives us the opportunity to test some of the interesting new agents now being developed and decipher whether tumors that have multiple mutations in these pathways are more or less susceptible to such agents," Baker said. By understanding the pathways that tumor cells depend on, scientists hope to develop better tools for predicting patient response to these same drugs.