October 4, 2011 feature
The brain on drugs: Defining the neural anatomy and physiology of morphine on dopamine neurons
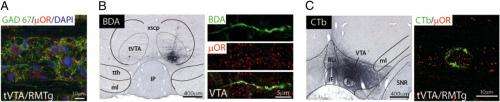
(Medical Xpress) -- Morphine's analgesic properties are as potent as its addictive potential are problematic. The neural pathway for that addiction is typically associated with dopamine (DA) neurons of the ventral tegmental area (VTA), despite the fact that the specific neuronal mechanisms involved are not well articulated. Recently, however, research conducted at the Université de Bordeaux and Université de Strasbourg in France found that morphine increases the firing of dopamine neurons by activating μ opioid receptor (μOR) receptors on the rostromedial tegmental nucleus (the VTA's GABAergic tail) – and that there is no morphine-induced activation of dopamine neurons in the absence of tonic VTA glutamatergic modulation.
The lead researcher, Marion Jalabert, Dr François Georges, and the multidisciplinary research team used in vivo electrophysiology, tract-tracing experiments, and targeted neuronal inactivation to successfully capture morphine activity in dopaminergic rat neurons. The project was made possible by collaboration between two groups with their own domain of expertise – in vivo electrophysiology by Georges’ team in Bordeaux and neuroanatomy by Michel Barrot’s group in Strasbourg.
The main challenge they faced, notes Georges, was to work in an intact in vivo preparation in order to dissect the neuronal circuit responsible for the excitatory effect of morphine on dopamine neurons. “To test our hypothesis that morphine will disrupt the excitatory/inhibitory balance in dopamine neurons we had to pharmacologically disconnect two closely-located brain structures. Another challenge,” he continues, “was to identify the molecular target of morphine onto the GABAergic tVTA/RMTg terminals innervating the VTA.”
To address these challenges, Georges explains, the groups had to develop highly-targeted drug application methods to be able to record in vivo activity of dopamine neurons and at the same time apply drugs in their vicinity – something made possible by the use of double-barrel pipette. “The key insight,” he adds, “was to preserve the integrity of the neuronal network.”
Georges identifies the next innovation to be developed and applied to the current experimental design as the use of optogenetic approaches to be able to activate or inhibit on demand the population of tVTA/RMTg neurons projecting to the VTA. Moreover, he points out, “This optogenetic approach could be coupled to electrophysiological recordings in freely moving animals. This will allow us to evaluate the impact of the context on the excitatory effect of morphine on dopamine neurons.”
Another innovative step will be developing virtual models. “Based on our findings and the previous in vitro findings obtained in 1992 by Johnson and North, it will be possible and really interesting to elaborate a computational model of the impact of morphine on the regulation of dopamine neuron excitability properties. This in silico approach will help to predict the effect of various drugs of abuse on the dopamine neurons depending on their excitability state.”
In terms of substance-dependence prevention and treatment, Georges cites the morphine-induced excitation of dopamine neurons that resulted from simultaneous NMDA/AMPA receptor activation and cessation of GABA receptor activation. “Our findings suggest that the intrinsic excitability of VTA-DA neurons may be important for scaling morphine responses.” Given that morphine has a high addictive potential in specific contexts, this indicates that the excitatory context of dopamine neurons may tune the addictive potency of morphine.
“Our research,” Georges stresses, “is currently focusing on understanding how the context and the history of the animal” – that is, exposure to stress or to other drugs of abuse tune in vivo the intrinsic excitability of VTA-DA neurons – “may be important for scaling morphine responses.”
Georges also sees their research having implications for a range of medical applications. “Our study,” he concludes, “provides a better understanding of tVTA/RMTg neurophysiology. The impact of this newly discovered brain area on dopamine neurons may provide new neuroanatomical targets for treatment. This neuronal participates to the etiology of neurological and psychiatric diseases, such as addiction, schizophrenia, mood disorders or Parkinson’s disease.”
More information: Neuronal circuits underlying acute morphine action on dopamine neurons. Published online before print September 19, 2011, doi: 10.1073/pnas.1105418108, PNAS September 27, 2011 vol. 108 no. 39 16446-16450
Related: Opioids excite dopamine neurons by hyperpolarization of local interneurons. SW Johnson and RA North, The Journal of Neuroscience, 1 February 1992, 12(2): 483-488
Copyright 2011 PhysOrg.com.
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of PhysOrg.com.