Device designed to treat a leading cause of blindness
(Medical Xpress) -- Every year, more than 200,000 people in the U.S. are diagnosed with Wet Age-Related Macular Degeneration, a leading cause of blindness in Americans age 60 or older. There is no known cure for the disease, which can lead to partial or complete vision loss.
But a new investigational device, invented by a University of Arizona professor, could dramatically change the way the degenerative disease is treated.
Wet Age-Related Macular Degeneration, or Wet AMD, occurs when abnormal blood vessels begin to grow behind the eye's macula, located in the center of the retina, and leak fluid that causes the macula to shift from its normal position and become damaged. It is more serious than the more common Dry Age-Related Macular Degeneration, which occurs when light-sensitive cells in the macula break down, blurring central vision.
One of the most common treatments for Wet AMD is repeated injections into the eye of anti-VGEF (anti-Vascular Endothelial Growth Factor) drugs, designed to slow the growth of abnormal blood vessels.
But those injections, administered as often as once a month for life, can be uncomfortable for patients and are not effective for everyone, said Russell Hamilton, radiation oncology professor and head of the physics section of the UA department of radiation oncology.
So Hamilton set out to find a less invasive way to treat patients that would reduce, or perhaps altogether eliminate, the need for repeated anti-VGEF injections.
His investigational device, which is now in the early stages of human study, is designed to deliver targeted radiation to blood vessel leaks via a small radioactive seed inserted behind the eye. The idea is that targeted radiation will provide a more long-term solution and help increase the effectiveness of initial anti-VGEF injections.
"When you're diagnosed with Wet AMD, you would have the anti-VGEF injection immediately. We're hoping the radiation will then increase the durability of the treatment, so that after the first month patients won't need to be injected again," said Hamilton, who also is a co-founder of Salutaris Medical Devices Inc., a privately held Tucson company founded to develop an investigational ophthalmic treatment for Wet AMD.
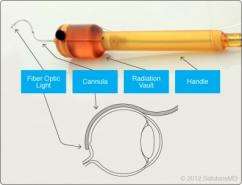
The device, about six inches in length, includes a wire with a curved end, designed to fit around the eye. It is inserted over the top of the eye, through a small incision, and moved toward the back of the eye, where it delivers a dose of radiation to repair troublesome leaks.
Requiring only a small incision, the procedure is minimally invasive and is intended to be done under local anesthesia in approximately 15 minutes in a physician's office or outpatient setting.
Researchers say it could be a safer, simpler alternative to external radiation, which also has been used to treat Wet AMD, and to other investigational treatments that involve penetration of the eye to deliver radiation.
"If this is proven in a larger trial, it's going to be game changing," said Dr. Baldassarre Stea, professor and chairman of the UA department of radiation oncology and co-principal investigator of the device study, along with Dr. Reid Schindler, clinical associate professor in the UA department of ophthalmology and vision science.
"Not only is it going to be beneficial from an economic point of view, in an era where resources are limited and the injections cost $2,000 a shot, but the quality of life of the patient will also be impacted," Stea said. "You regain your vision better, you don't have to go back and forth to the ophthalmologist every month to get the injection, and you save money and aggravation."
Hamilton said he began working on his device after learning of the successful use of radiation to treat melanoma of the eye.
In January, the results of a Phase 1 study to evaluate the safety of the device were shared during Retina Meeting 2012. Of the six test subjects treated with the device, all saw improvement in vision, none experienced additional vision loss and three were able to discontinue their anti-VGEF injections. Furthermore, there were no reported "Serious Adverse Events" or "Unanticipated Adverse Device Effects."
Additional studies are expected to get under way later this year. Stea said if those go well, FDA approval for the device could happen within a few years.