The RHAU helicase: A key player in blood formation
Scientists at the Friedrich Miescher Institute for Biomedical Research have discovered that the helicase RHAU, a protein that can resolve complex structures in both DNA and RNA molecules, is essential for early embryonic development. Mice that specifically lack RHAU in haematopoietic stem cells – which ultimately give rise to all cellular components of blood – develop severe anaemia. The underlying causes are a dramatically reduced half-life of red blood cells as well as impaired cell division of haematopoietic progenitor cells.
EPO, in full erythropoietin, has gained notoriety, as certain athletes follow strenuous practice routines to boost their body’s own EPO levels, or go even beyond what is fair in sports. This is in stark contrast to mice that lack the RHAU helicase in blood precursor cells who develop seemingly effortlessly and without fault an astounding 800 fold increase of their EPO levels. However, while EPO drives the production of mature red blood cells and thus helps to improve physical performance in sportsmen, Rhau-null mice paradoxically suffer from anaemia.
In a process called haematopoiesis, all cellular blood components, namely red and white blood cells, derive from haematopoietic stem cells (HSC), which predominantly reside in the bone marrow. The DEAH helicase RHAU, first described by the group of Yoshi Nagamine at the Friedrich Miescher Institute for Biomedical Research, is highly expressed in lymphoid and bone marrow cells. It is the only known helicase that can resolve both RNA and DNA G-quadruplex structures (G4). G4 structures naturally occur in guanine-rich DNA and RNA stretches, and seem to play a role in gene regulation, as recent evidence suggests.
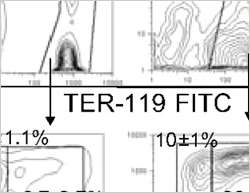
To assess the biological function of RHAU in haematopoiesis, the former FMI PhD student Janice Lai and her colleagues at the Friedrich Miescher Institute for Biomedical Research worked with mice that lacked the RHAU protein in HSCs and in all cells derived thereof. These mice developed severe anaemia with drastically reduced levels of red blood cells and haemoglobin levels. Bone marrow transplantation experiments revealed that the observed defect was caused by the blood precursor cells themselves rather than their environment. The scientists subsequently discovered that in the absence of RHAU, the half-life of mature red blood cells was reduced by 50%, accounting at least in part for the observed anaemia. As a consequence of the anaemia, EPO levels rise to boost the production of red blood cells. Accordingly, in Rhau-null mice EPO levels skyrocketed to an 800 fold increase, but to no avail. While the earliest progenitor cell population in the formation of red blood cells, termed ProE, was strongly increased, progenitor cell numbers declined with every step during the maturation of erythrocytes. Thus, in the absence of RHAU and despite the high levels of EPO, the differentiation and maturation process of red blood cells was dramatically impaired. While the differentiation defect was most prominent at the ProE stage, it was also present in other progenitor cell populations including those giving rise to white blood cells. Additional bone marrow transplantation experiments revealed that wild type HSCs readily out-competed Rhau-null HSCs to reconstitute both red and white blood cells. These findings further supported the hypothesis that RHAU is essential for haematopoietic progenitor expansion and their differentiation potential in a cell autonomous manner.
Lastly, the scientists discovered that ProE lacking RHAU proliferate at substantially lower rates. Transcriptome analysis of wild type and RHAU-deficient ProE cells revealed that a significant fraction of RHAU-regulated genes contain a G4 motif in their promoter region. Therefore, the authors of the study conclude that RHAU may regulate transcription of a distinct set of genes via its DNA G4 resolvase activity.
Taken together, this study provides the first piece of evidence that a DEAH helicase is essential for haematopoiesis in mammals.
More information: Lai JC, et al. (2012) The DEAH-box helicase RHAU is an essential gene and critical for mouse hematopoiesis. Blood 119:4291-300