Collaboration finds kidney disease tied to DNA damage
(Medical Xpress) -- A research collaboration involving Rockefeller University and more than two dozen other institutions has found a link between a gene mutation and chronic kidney failure. The study, published in Nature Genetics in July, found patients who had a specific kind of kidney disease — called karyomegalic interstitial nephritis — were likely to also have a mutation on a particular gene, FAN1, which codes for a protein that helps fix DNA damage.
Agata Smogorzewska, assistant professor at Rockefeller and an expert on FAN1, worked with her lab to provide evidence of a causal relationship between the mutation and the disease.
Kidney disease is a health problem that has grown ever more prevalent in recent decades, and it can be due to a number of factors. The most common today is type 2 diabetes, which, with an increasingly overweight population, helps explain the recent rise of kidney problems. This study brings light to a new, genetic factor in the complex picture of renal failure.
Friedhelm Hildebrandt, a professor of pediatrics and human genetics at the University of Michigan, was sequencing the genes of a group of families with a history of kidney disease last year when he came across the FAN1 mutation. His lab found that FAN1 was mutated in 9 out of the 10 families with karyomegalic interstitial nephritis.
Smogorzewska, head of the Laboratory of Genome Maintenance at Rockefeller, joined Hildebrandt in a collaboration to draw out the details of this possible genetic cause.
“Our lab is very interested in this gene and others that are involved in DNA damage, and we have the tools to study them, so we were eager to join Hildebrandt’s lab in understanding how these patients’ cells behaved,” Smogorzewska says.
The relationship between FAN1 and kidney disease was an exciting and surprising finding for Smogorzewska: she first identified FAN1 as a postdoc at Harvard Medical School. But she wasn’t looking at the kidney — she was studying Fanconi anemia, a genetic disease that interferes with a cell’s ability to repair DNA damage, leading to cancer, bone marrow failure and other health problems.
DNA damage by itself is an everyday occurrence for cells: as many as 1 million so-called lesions can exist in the DNA of each cell each day. If the person is healthy, the cells identify and repair the damage. For others, the damage goes unchecked, leading to a dangerous environment in the body that has been implicated in several disorders, including Fanconi anemia, as well as cancer and premature aging.
After identifying FAN1, Smogorzewska found that it helps repair DNA damage, similar to genes implicated in Fanconi anemia. But a study of the genomes of patients with the disease revealed that none of them had a mutation in FAN1.
Smogorzewska went on to identify other genes that had a causal relationship with Fanconi anemia, but uncovering the functions of FAN1 remained a focus of her research.
In the present study, Smogorzewska’s lab used its FAN1 expertise to investigate whether there was a causal relationship between the gene mutation and kidney disease.
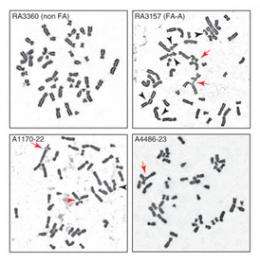
One test took cells from the patients and exposed them to mitomycin C, a drug often used in chemotherapy. Mitomycin C causes crosslinking in DNA, which is where both strands of the double helix become bonded together in such a way that they can’t separate, rendering them unable to replicate and create new cells. FAN1 is known to repair this DNA damage, and when the lab looked at the patients’ cells in the presence of the drug, they saw significant damage, meaning FAN1 wasn’t working properly. When the corrected version of the FAN1 gene was placed back in these cells, their ability to repair the crosslinking damage was restored.
“This is our proof that we’ve likely identified the right gene: the ability of the FAN1 protein to restore the cells’ function. Additionally, if you find multiple families with the same phenotype and the same gene being mutated, it’s a really good indication that you have the right gene,” says Smogorzewska.
Though they may have found the gene, there is still much to learn about the link between DNA damage and kidney failure. What’s causing the DNA damage in the first place, leaving those with the mutated gene unable to repair it? And why does the damage target the kidney, but spare other organs? Smogorzewska’s lab is now addressing these questions.
“Often we get clues from diseases into general biology that will be applicable to everybody, because the disease points us in the direction we should be looking at,” Smogorzewska says.
More information: FAN1 mutations cause karyomegalic interstitial nephritis, linking chronic kidney failure to defective DNA damage repair, Nature Genetics 44: 910–915 (July 8, 2012)