Pioneering heart disease treatment
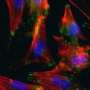
(Medical Xpress) -- Researchers at King's College London have developed the first artificial functioning blood vessel outside of the body, made from reprogrammed stem cells from human skin. The team also saw the cells develop into a blood vessel inside the body for the first time.
The new technique could have real potential to treat patients with heart disease – the biggest killer in the UK – by either injecting the reprogrammed cells into the leg or heart to restore blood flow or grafting an artificially developed vessel into the body to replace blocked or damaged vessels. The treatment could also benefit diabetic patients with poor circulation, preventing leg amputation.
Stem cell therapy to treat heart disease is already being carried out in the clinic using bone marrow cells, however, the long-term effectiveness at the moment is minimal and some types of stem cells have the potential to become a tumour after they are introduced into the body. Published in the journal Proceedings of the National Academy of Sciences, this new study demonstrates that a new type of partial stem cell developed from fibroblasts (skin cells) can be reprogrammed into vascular cells before going into the body, which have no risk turning into tumours.
The King’s team introduced four genes to human fibroblasts in the laboratory to reprogramme them into partial stem cells so they could become vascular cells. When these newly created cells were injected into an ischemic leg (a leg with restricted blood flow) in an animal model, the function of the leg was improved.
The process of developing vascular cells from skin cells took two weeks, which makes a personalised approach of turning a patient’s own skin cells into vascular cells feasible for treatment of vessel-blocking related diseases. The researchers say the next step is to test this approach in cells from patients with vascular disease.
Professor Qingbo Xu, British Heart Foundation and John Parker Chair at King’s College London, said: "This is very exciting research – we are thrilled to have developed the first artificial functioning blood vessel outside of the body made from stem cells derived from human fibroblasts."
"Heart disease is the biggest killer in this country, so if we can develop this approach as personalised treatments for patients with the condition, it will be a significant step forward. This is an early study and more research needs to be done into how this approach works in patients, but the aim is to be able to inject reprogrammed cells into areas of restricted blood flow, or even graft an entirely new blood vessel into a patient to treat serious cardiovascular diseases."
Dr Hélène Wilson, Research Advisor at the British Heart Foundation, which co-funded the study, said: ‘This breakthrough is an exciting step forward in the field of regenerative medicine, but further studies and safety checks will need to be carried out before we know if it will help lead to new treatments for patients.
"This team showed they can derive so-called ‘partial pluripotent’ cells from human skin cells in just four days, and convert them directly into a type of cell that lines our blood vessels. Traditional methods take longer and come with an increased chance of tumours forming from the new cells."
"The discovery could help lead towards future therapies to repair hearts after they are damaged by a heart attack. As well as playing a part in a possible future regenerative treatment, these cells might also be used in drug screening to find new treatments to tackle inherited diseases."
More information: www.pnas.org/content/early/201 … 526109.full.pdf+html