Studies: Alzheimer drug may stabilize brain plaque (Update)
An experimental drug that failed to stop mental decline in Alzheimer's patients in the U.S. and Canada also showed some potential benefit in slowing brain plaque, fuller results of two major studies show.
Some patients on the drug had stable levels of brain plaque and less evidence of nerve damage compared to others who were given a dummy treatment, researchers reported Tuesday in Sweden.
About 35 million people worldwide have dementia, and Alzheimer's is the most common type. Current medicines such as Aricept and Namenda just temporarily ease symptoms. There is no known cure.
The drug, called bapineuzumab, is made by Pfizer Inc. and Johnson & Johnson. The new results suggest it might work if given earlier in the course of the disease, before so much damage and memory loss have occurred that it might not be possible to reverse, experts say.
"We're very disappointed that we were not able to come up with a treatment to provide to our dementia patients in the near term," said Dr. Reisa Sperling, director of the Alzheimer's center at Brigham and Women's Hospital in Boston and leader of one of the studies.
But brain imaging and spinal fluid tests are "very encouraging" and suggest "that we were doing something to the biology of the disease" by giving the drug, she said.
"We've got a path forward" now to test it in people with mild mental impairment or those who show plaque on brain imaging but have not yet developed symptoms of dementia, Sperling said.
Of people with mild cognitive impairment, about 15 to 20 percent a year will develop Alzheimer's disease.
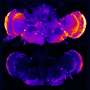
Bapineuzumab is designed to attach to and help clear amyloid, the stuff that makes up the sticky plaque that clogs patients' brains, harming nerve cells and impairing memory and thought. Doctors don't know whether amyloid is a cause or just a symptom of Alzheimer's, but many companies are testing drugs to try to remove it.
Two studies of more than 1,000 patients each in the United States and Canada tested bapineuzumab in people with mild to moderate Alzheimer's. Sperling's study involved people with a gene that raises the risk of developing the disease. Dr. Stephen Salloway, a neurologist at Brown Medical School, led the other study of people without the gene.
Both researchers have consulted for the companies that make the drug and presented results Tuesday at a neurology conference in Sweden.
The companies previously announced that the 18-month studies failed to meet their main goal of slowing mental decline or improving activities of daily living.
However, brain imaging on a subset of patients in Sperling's study found 9 percent less amyloid in those on bapineuzumab compared to those on a dummy treatment. The drug group had stable levels while the others developed more plaque. Spinal fluid tests on some participants also showed the drug group had less of another substance that is released when nerve cells are damaged.
"I found that very encouraging, that we were doing something to the biology of the disease. We're having an impact on nerve cell injury," Sperling said.
There were potential safety concerns. There were 15 deaths among the 673 in bapineuzumab group versus only five among the 448 in the placebo group. Six of the deaths in the drug group were from various forms of cancer. But a wider review of thousands of patients in multiple studies of bapineuzumab found that cancer was not more common among those on the drug.
The cancer deaths "were wide and varied, and they weren't a specific type of cancer, so that's not raising any red flags," said an independent expert, Dr. Maria Carrillo, a senior scientist at the Alzheimer's Association.
Salloway's study produced less evidence of benefit. Too few participants had brain imaging to make definitive conclusions about amyloid, and there was just a trend toward less of the nerve-damage substance in the group receiving the higher of two doses tested.
Bapineuzumab is given as periodic intravenous infusions, and the companies have said they are stopping development of that form but continuing to test a version that can be given as a shot.
More results on this drug and a similar one—Eli Lilly & Co.'s solanezumab—will be presented at a conference in Boston next month. Lilly recently announced that combined results of two large studies of solanezumab suggested some benefit on cognition.
Copyright 2012 The Associated Press. All rights reserved. This material may not be published, broadcast, rewritten or redistributed.