Study sheds light on bone marrow stem cell therapy for pancreatic recovery
Researchers at Cedars-Sinai's Maxine Dunitz Neurosurgical Institute have found that a blood vessel-building gene boosts the ability of human bone marrow stem cells to sustain pancreatic recovery in a laboratory mouse model of insulin-dependent diabetes.
The findings, published in a PLOS ONE article of the Public Library of Science, offer new insights on mechanisms involved in regeneration of insulin-producing cells and provide new evidence that a diabetic's own bone marrow one day may be a source of treatment.
Scientists began studying bone marrow-derived stem cells for pancreatic regeneration a decade ago. Recent studies involving several pancreas-related genes and delivery methods – transplantation into the organ or injection into the blood – have shown that bone marrow stem cell therapy could reverse or improve diabetes in some laboratory mice. But little has been known about how stem cells affect beta cells – pancreas cells that produce insulin – or how scientists could promote sustained beta cell renewal and insulin production.
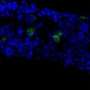
When the Cedars-Sinai researchers modified bone marrow stem cells to express a certain gene (vascular endothelial growth factor, or VEGF), pancreatic recovery was sustained as mouse pancreases were able to generate new beta cells. The VEGF-modified stem cells promoted growth of needed blood vessels and supported activation of genes involved in insulin production. Bone marrow stem cells modified with a different gene, PDX1, which is important in the development and maintenance of beta cells, resulted in temporary but not sustained beta cell recovery.
"Our study is the first to show that VEGF contributes to revascularization and recovery after pancreatic injury. It demonstrates the possible clinical benefits of using bone marrow-derived stem cells, modified to express that gene, for the treatment of insulin-dependent diabetes," said John S. Yu, MD, professor and vice chair of the Department of Neurosurgery at Cedars-Sinai, senior author of the journal article.
Diabetes was reversed in five of nine mice treated with the injection of VEGF-modified cells, and near-normal blood sugar levels were maintained through the remainder of the six-week study period. The other four mice survived and gained weight, suggesting treatment was beneficial even when it did not prompt complete reversal. Lab studies later confirmed that genetically-modified cells survived and grew in the pancreas and supported the repopulation of blood vessels and beta cells.
Anna Milanesi, MD, PhD, working in Yu's lab as an endocrinology fellow, is the article's first author. The researchers cautioned that although this and other related studies help scientists gain a better understanding of the processes and pathways involved in pancreatic regeneration, more research is needed before human clinical trials can begin.
Insulin-dependent diabetes occurs when beta cells of the pancreas fail to produce insulin, a hormone that regulates sugar in the blood. Patients must take insulin injections or consider transplantation of a whole pancreas or parts of the pancreas that make insulin, but transplantation carries the risk of cell rejection.
More information: PLOS ONE paper is titled "Beta-cell Regeneration Mediated by Human Bone Marrow Mesenchymal Stem Cells."