Scientists provide detailed view of brain protein structure: Results may help improve drugs for neurological disorders
Researchers have published the first highly detailed description of how neurotensin, a neuropeptide hormone which modulates nerve cell activity in the brain, interacts with its receptor. Their results suggest that neuropeptide hormones use a novel binding mechanism to activate a class of receptors called G-protein coupled receptors (GPCRs).
"The knowledge of how the peptide binds to its receptor should help scientists design better drugs," said Dr. Reinhard Grisshammer, a scientist at the NIH's National Institute of Neurological Disorders and Stroke (NINDS) and an author of the study published in Nature.
Binding of neurotensin initiates a series of reactions in nerve cells. Previous studies have shown that neurotensin may be involved in Parkinson's disease, schizophrenia, temperature regulation, pain, and cancer cell growth.
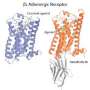
Dr. Grisshammer and his colleagues used X-ray crystallography to show what the receptor looks like in atomic detail when it is bound to neurotensin. Their results provide the most direct and detailed views describing this interaction which may change the way scientists develop drugs targeting similar neuropeptide receptors.
X-ray crystallography is a technique in which scientists shoot X-rays at crystallized molecules to determine a molecule's shape and structure. The X-rays change directions, or diffract, as they pass through the crystals before hitting a detector where they form a pattern that is used to calculate the atomic structure of the molecule. These structures guide the way scientists think about how proteins work.
Neurotensin receptors and other GPCRs belong to a large class of membrane proteins which are activated by a variety of molecules, called ligands. Previous X-ray crystallography studies showed that smaller ligands, such as adrenaline and retinal, bind in the middle of their respective GPCRs and well below the receptor's surface. In contrast, Dr. Grisshammer's group found that neurotensin binds to the outer part of its receptor, just at the receptor surface. These results suggest that neuropeptides activate GPCRs in a different way compared to the smaller ligands.
Forming well-diffracting neuropeptide-bound GPCR crystals is very difficult. Dr. Grisshammer and his colleagues spent many years obtaining the results on the neurotensin receptor. During that time Dr. Grisshammer started collaborating with a group led by Dr. Christopher Tate, Ph.D. at the MRC Laboratory of Molecular Biology, Cambridge, England. Dr. Tate's lab used recombinant gene technology to create a stable version of the neurotensin receptor which tightly binds neurotensin. Meanwhile Dr. Grisshammer's lab employed the latest methods to crystallize the receptor bound to a short version of neurotensin.
The results published today are the first X-ray crystallography studies showing how a neuropeptide agonist binds to neuropeptide GPCRs. Nonetheless, more work is needed to fully understand the detailed signaling mechanism of this GPCR, said Dr. Grisshammer.
More information: White et al., "Structure of the agonist-bound neurotensin receptor." Nature, published online October 10, 2012. DOI: 10.1038/nature11558