Gene therapy cocktail shows promise in long-term clinical trial for rare fatal brain disorder
Results of a clinical trial that began in 2001 show that a gene therapy cocktail conveyed into the brain by a molecular special delivery vehicle may help extend the lives of children with Canavan disease, a rare and fatal neurodegenerative disorder.
A report of the trial appears in the Dec. 19, 2012 online edition of the journal Science Translational Medicine.
The form of gene therapy was created and developed at the University of North Carolina School of Medicine. The work was spearheaded by R. Jude Samulski, PhD, a study senior author, professor of pharmacology and director of UNC's Gene Therapy Center. The treatment uses a virus (adeno-associated virus, or AAV) as a "viral vector" meticulously tailored to enter the brain and safely switch good genes for bad.
"This was the first AAV-based gene therapy produced by a U.S. academic institution to be approved for neurological use by the FDA," Samulski said. "It's also the first vector produced by the university's Gene Therapy Center Vector Core facility to go into patients."
Children with Canavan disease have mutations in the ASPA gene that normally codes for an enzyme that helps the brain degrade N-acetyl-aspartate (NAA). The unregulated buildup of NAA is toxic to the brain's gray matter, the protective myelin sheath surrounding nerve cells. As the myelin deteriorates and neurons becoming unable to communicate, the child's head size increases (macrocephaly), there are movement problems such as an inability to crawl, seizures occur, vision becomes impaired, and the children often die within three years of age. Fewer than 1,000 children in the U.S. have the disorder.
Samulski arrived at UNC in 1993 to establish the UNC Gene Therapy Center and has long pioneered methodologies for using viruses to deliver genes effectively and safely to various targets in the body, including the brain, lungs, liver, heart, and muscle. As a graduate student at the University of Florida in the early 1980s, his thesis project was understanding and developing AAV as a vector for delivering therapeutic genes which has help launch this new field of molecular medicine. This work eventually led to development of AAV type 2, which has been used for gene therapy vector trials in cystic fibrosis, hemophilia, Parkinson's diseases, retinal disorders, and in several other settings, including the first gene therapy clinical trial for muscular dystrophy in the U.S developed by Dr. Samulski and his first graduate student, Dr. Xiao Xiao, professor in the UNC Eshelman School of Pharmacy.
In this Canavan disease phase 1/2 safety study, 13 children were treated at the Cell and Gene Therapy Center at the University of Medicine and Dentistry of New Jersey (UMDNJ) in Stratford, N.J. Principal Investigator and first author of the study is Paola Leone, PhD, associate professor of cell biology at UMDNJ. The children were treated in 2001, 2003 and 2005, corresponding to AAV vector production runs. Their ages at the time of treatment ranged from four to 83 months.
Working with Samulski's UNC lab colleagues, Leone's neurosurgical team used MRI imaging to guide them to the proper location and depth in the lateral ventricle of the brain for inserting six very thin catheters via small holes drilled in the skull.
The team then pumped in a solution carrying the vector package containing the replacement ASPA gene. This amounted to about 900 billion genomic particles of replacement gene held by the AAV vector – roughly the size of a quarter – that were pumped into each of the six catheter sites. The catheters were then removed.
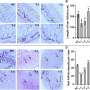
Following the treatments, the patients went home with their families and were tracked with behavioral tasks and brain imaging studies. The investigators found that the gene therapy was safe and has led to a decrease in NAA in the brain, together with decreased seizure frequency and "clinical stabilization," the greatest observed in youngest patients, those treated before 2 years of age. These include improvements in attention, sleep, and greater degree of movement improvements when lying down and rolling.
"As the trial continued, the FDA let us go to younger and younger patients," Samulski said. "We were successful in being able to treat a 3-months-old infant who was diagnosed in utero … and that child is alive today and is the youngest person who has ever been treated with gene therapy."
The UNC scientist views the study a definite success from the safety perspective. "The genetic information put into the brains of individuals has not caused adverse effects, toxicity, or cancer. It also has great potential efficacy for treating other degenerative neurological disorders, including Parkinson's and Alzheimer's diseases."