Cell loss in the brain relates to variations in individual symptoms in Huntington's disease
Scientists have wrestled to understand why Huntington's disease, which is caused by a single gene mutation, can produce such variable symptoms. An authoritative review by a group of leading experts summarizes the progress relating cell loss in the striatum and cerebral cortex to symptom profile in Huntington's disease, suggesting a possible direction for developing targeted therapies. The article is published in the latest issue of the Journal of Huntington's Disease.
Huntington's disease (HD) is an inherited progressive neurological disorder for which there is presently no cure. It is caused by a dominant mutation in the HD gene leading to expression of mutant huntingtin (HTT) protein. Expression of mutant HTT causes subtle changes in cellular functions, which ultimately results in jerking, uncontrollable movements, progressive psychiatric difficulties, and loss of mental abilities.
Although it is caused by a single gene, there are major variations in the symptoms of HD. The pattern of symptoms shown by each individual during the course of the disease can differ considerably and present as varying degrees of movement distrubances, cognitive decline, and mood and behavioral changes. Disease duration is typically between ten and twenty years.
Recent investigations have focused on what the presence of the defective gene does to various structures in the brain and understanding the relationship between changes in the brain and the variability in symptom profiles in Huntington's disease.
Analyses of post-mortem human HD tissue suggest that the variation in clinical symptoms in HD is strongly associated with the variable pattern of neurodegeneration in two major regions of the brain, the striatum and the cerebral cortex. The neurodegeneration of the striatum generally follows an ordered and topographical distribution, but comparison of post-mortem human HD tissue and in vivo neuroimaging techniques reveal that the disease produces a striking bilateral atrophy of the striatum, which in these recent studies has been found to be highly variable.
"What is especially interesting is that recent findings suggest that the pattern of striatal cell death shows regional differences between cases in the functionally and neurochemically distinct striosomal and matrix compartments of the striatum which correspond with symptom variation," says author Richard L.M. Faull, MB, ChB, PhD, DSc, Director of the Centre for Brain Research, University of Auckland, New Zealand.
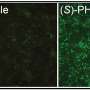
"Our own recent detailed quantitative study using stereological cell counting in the post-mortem human HD cortex has complemented and expanded the neuroimaging studies by providing a cortical cellular basis of symptom heterogeneity in HD," continues Dr Faull. "In particular, HD cases which were dominated by motor dysfunction showed a major total cell loss (28% loss) in the primary motor cortex but no cell loss in the limbic cingulate cortex, whereas cases where mood symptoms predominated showed a total of 54% neuronal loss in the limbic cingulate cortex but no cell loss in the motor cortex. This suggests that the variable neuronal loss and alterations in the circuitry of the primary motor cortex and anterior cingulate cortex associated with the variable compartmental pattern of cell degeneration in the striatum contribute to the differential impairments of motor and mood functions in HD."
The authors note that there are still questions to be answered in the field of HD pathology, such as, how and when pathological neuronal loss occurs; whether the progressive loss of neurons in the striatum is the primary process or is consequential to cortical cell dysfunction; and how these changes relate to symptom profiles.
"What is clear however is that the diverse symptoms of HD patients appear to relate to the heterogeneity of cell loss in both the striatum and cerebral cortex," the authors conclude. "While there is currently no cure, this contemporary evidence suggests that possible genetic therapies aimed at HD gene silencing should be directed towards intervention at both the cerebral cortex and the striatum in the human brain. This poses challenging problems requiring the application of gene silencing therapies to quite widespread regions of the forebrain which may be assisted via CSF delivery systems using gene suppression agents that cross the CSF/brain barrier."
More information: "New Perspectives on the Neuropathology in Huntington's Disease in the Human Brain and its Relation to Symptom Variation," by Henry J. Waldvogel, Eric H. Kim, Doris C.V. Thu, Lynette J. Tippett, and Richard L.M. Faull. Journal of Huntington's Disease, Volume 1/Issue 2 (December 2012), DOI 10.3233/JHD-2012-120018