Researchers discover that errors in RNA splicing lead to a class of neurological disorders
(Medical Xpress)—Researchers have found that missteps in a basic cellular process, RNA splicing, is the culprit behind a class of rare neurological disorders manifested by intellectual disability and stunted development.
In RNA splicing, nascent RNA molecules are modified and edited so they can then go about the business of synthesizing proteins. Incorrect splicing of RNA, however, impairs cellular function.
Qingqing Wang, a doctoral student in systems biology at Harvard Medical School, found that genome-wide splicing errors were caused by mutations in a gene called PQBP1, which encodes a protein that has previously been linked to Renpenning syndrome, an X-linked cognitive disorder.
"This is one of the first studies to show the effect of RNA processing on neural defects across the whole genome," said Pamela Silver, HMS professor of systems biology and senior study author.
These findings were published on March 15 in the journal Genes and Development.
Intellectual Disability Disease comprises of a group of rare genetic disorders that cause a series of neurological defects. In particular, Wang and Silver studied Renpenning syndrome, a disease characterized by an unusually small head circumference, mental disability and trouble with movement and coordination. Since it is genetic and rare, only about 10 to 15 families worldwide are known to have this disease.
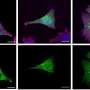
RNA splicing is one of the many ways the human genome achieves diversity. In some cases, though, this diversity comes at a price. In the case of this particular disorder, when mutations in PQBP1 caused incorrect RNA splicing, the outcome was stunted growth of neuronal dendrites, the tree-like structures at the ends of neurons that conduct electrical stimulation.
Wang came across the PQBP1 protein while screening for molecules that affected RNA splicing in apoptosis, or cell death. Upon further investigation within neurons in particular, Wang was able to isolate more than 500 different splicing events associated with PQBP1. With nearly all these events, Wang found functional defects downstream.
"It was really heroic, the work that Wang conducted, since she's never before worked with neurons," said Silver.
The neurons were provided by Michael Greenberg, the Nathan Marsh Pusey Professor of Neurobiology at HMS and the Department Chair of Neurobiology. According to Wang, "I had a seven- to fourteen-day window with a batch of cells and the tools had to be very refined in order to work with them."
This new frontier for Wang also yielded a technical first: Wang developed a new genome-wide method to analyze how the process of alternative RNA splicing can be affected by various factors.
Researchers were particularly surprised that the malfunctioning of PQBP1, and hence the cause of incorrect RNA splicing, pointed to a gene called NCAM1.
"The resulting RNA can be spliced in two different ways," explained Wang, "each creating a different type of protein, and each protein differing from the other by only about ten amino acids."
If the splicing creates the shorter version of the protein, neurons generate more and longer dendrites. If the longer version of the protein is produced, however, neurons generate fewer and shorter dendrites.
As such, if PQBP1 is mutated, it results in incorrect splicing downstream, especially causing the RNA encoded by NCAM1 to be spliced into the longer form. These errors result in defects in neuronal dendrites, a distinctive feature of intellectual disability disorders.
"This process is a success story for systems biology," said Silver. "The examination of the cell and its components as a whole and then narrowing down to the specific gene is one of the discipline's facets. We weeded through 600 to 700 affected genes and were able to show that NCAM1 was consistently the affected gene in question."
The next step would be to test other possible targets for the protein PQBP1. "This way, maybe we can identify a possible therapeutic target," said Wang. "But knowing the mechanisms behind rare diseases is beneficial in itself because they could be generalizable to other neurological diseases."
More information: genesdev.cshlp.org/content/27/6/615.abstract