March 27, 2013 feature
Separate lives: Neuronal and organismal lifespans decoupled
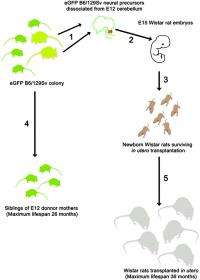
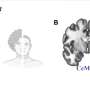
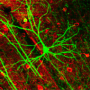
(Medical Xpress)—Replicative aging (also known as replicative senescence) causes mammalian cells to undergo a process of growth arrest dependent on telomeres (the shortening of repeated sequences at the ends of chromosomes). Neurons, on the other hand, are exempt from aging, and so the question of their actual lifespan has remained unanswered. Recently, however, scientists at the University of Pavia and the University of Turin demonstrated that neuronal lifespan is not limited by the organism's maximum lifespan but, remarkably, continues when transplanted in a longer-living host. The researchers accomplished this by transplanting embryonic mouse cerebellar precursors into the developing brain of longer-living rats, in which the grafted mouse neurons survived for up to three years – twice the average lifespan of the donor mice.
Dr. Lorenzo Magrassi discussed the challenges he and his colleagues, Dr. Ketty Leto and Dr. Ferdinando Rossi, encountered in their research. "Cell transplantation into the developing rat brain is a technique that was originally developed by us and other research groups in the early nineties of the last century," Magrassi tells Medical Xpress. "In recent years, we improved the protocol that, now standardized, allows reliable implantation rates with good survival rates." While not all implanted embryos develop into adult animals carrying a viable transplant, Magrassi adds, the percentage of those that do is sufficient to plan a long-term survival experiment involving roughly 100 such successfully-born animals.
In addressing these challenges, Magrassi says that together with the intrinsic bonus of studying cells inside the nervous system, which is immunoprivileged, they transplanted cells before development of the thymus (a specialized organ of the immune system) was complete. The latter can help induce immunological tolerance in the host to the engrafted cells.
One remaining question is if their research can potentially be extended to determine whether or not a maximum lifespan exists for any postmitotic mammalian cells – Including neurons. "Similar techniques can, in principle, be extended to other organs containing perennial cells," Magrassi notes, "but we don't have direct experience with injecting cells into organs outside of the central nervous system." Since the central nervous system is privileged compared to other organs that are more prone to immunological surveillance and attack, a major problem when transferring their experimental paradigm to other organs, he explains, could be an increase in immunological problems.
The scientists say their results suggest that neuronal survival and aging are coincidental but separable processes, thus increasing the hope that extending organismal lifespan by dietary, behavioral, and pharmacologic interventions will not necessarily result in a neuronally depleted brain. "Even after taking into account the obvious species differences, our results in rodents can be extrapolated by analogy to humans and other longer-living species where this sort of experiment is impossible," Magrassi explains. "Our findings suggest that extending life by extending average organismal lifespan – a hallmark of all technologically advanced societies – will not necessarily result in neuron-impoverished brains well before the longer-living individual dies." This bodes well for those studying life extension: Their efforts are not intrinsically futile, Magrassi notes, because in the absence of pathology, prolonging life span does not necessarily mean dementia due to widespread loss of neurons, as many people still think. "Roughly speaking," Magrassi illustrates, "if the average lifespan of humans is now 80 years, our results suggest that at ages up to 160 years our neurons can survive if not hit by specific insults.:
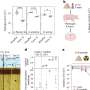
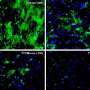
That said, however, Magrassi acknowledges that neuronal death is not the only effect of normal aging in the brain. "For example," he illustrates, "cerebellar neurons – which in term of synaptic loss behave like the majority of neurons in the brain – show a substantial loss of dendritic branches, spines and synapses in normal aging. In our research, we studied transplanted mouse Purkinje cells to determine if their spine density decreased with time at the same rate of Purkinje cells in the mouse or in the rat." Purkinje cells are large GABAergic (that is, gamma-Aminobutyric acid-producing) neurons, with many branching extensions, found in the cortex of the cerebellum. "The results of our experiments indicate that age-related progressive spine loss of grafted mouse Purkinje cells follows a slower pace, typical of the longer living rat, thus reaching absolute levels of spine loss comparable to those observed in aged mice at much longer survival times that are typical of the rat."
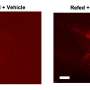
Moreover, Magrassi adds that their experiments clearly show that by escaping immunological rejection, transplanted neurons can survive undisturbed for the entire life of the host. "This has implications for the ongoing discussion of the detrimental effects of immune attacks on transplanted neural cells for therapeutic purposes,"
Moving forward, in order to screen for intra- and extracellular changes that could be responsible for the long term survival of the mouse cells transplanted into rat brains – as well as the slowdown of dendritic spine loss – the team is planning to perform host and transplanted cell microdissection followed by a proteomic approach. "If we discover what factor or factors cause those changes," Magrassi points out, "we could hopefully then develop more efficient drugs for treating all pathological neurodegenerative conditions in which neurons start to lose synaptic contacts and die well before organismal death – for example, dementia, memory loss and cognitive impairment. Of course," he adds, "this work is still in progress and the results are preliminary."
In addition, the scientists are currently testing xenotransplantation using different transgenic mouse strains with altered aging pathways as donors to characterize the pathways that led to their results.
Magrassi sees other areas of research that might benefit from their study. "Knowing that neuronal aging in rodents is not a cell-autonomous process is important not only for neuroscience," he concludes. "It also has implications for evolutionary biology and epidemiology."
More information: Lifespan of neurons is uncoupled from organismal lifespan, PNAS March 12, 2013 vol. 110 no. 11 4374-4379, doi:10.1073/pnas.1217505110
Copyright 2013 Medical Xpress
All rights reserved. This material may not be published, broadcast, rewritten or redistributed in whole or part without the express written permission of Phys.org.