Mapping of cancer cell fuel pumps paves the way for new drugs
For the first time, researchers at Karolinska Institutet in Sweden have managed to obtain detailed images of the way in which the transport protein GLUT transports sugars into cells. Since tumours are highly dependent on the transportation of nutrients in order to be able to grow rapidly, the researchers are hoping that the study published in the scientific magazine Nature Structural & Molecular Biology will form the basis for new strategies to fight cancer cells.
In order to be able to fuel their rapid growth, cancer tumours depend on transporter proteins to work at high speed to introduce sugars and other nutrients that are required for the cell's metabolism. One possible treatment strategy would therefore be to block some of the transporters in the cell membrane which operate as fuel pumps, thus starving out and killing the cancer cells.
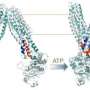
One important group of membrane transporters is the GLUT family, which introduces glucose and other sugars into the cell. Glucose is one of the most important energy sources for cancer cells and GLUT transporters have been shown to play a key role in tumour growth in many different types of cancer. In the current study, researchers from Karolinska Institutet have performed a detailed study of the way in which suger transport is executed by the protein XylE, from the Escherichia coli bacterium, whose function and structure is very similar to GLUT transporters in humans. For the first time, the researchers have described the way in which the protein's structure changes between two different conformations when it binds and transports a sugar molecule.
"In showing details of the molecular structure of the region that bind the sugar, our study opens up the opportunities to more efficiently develop new substances that may inhibit GLUT transporters", says Pär Nordlund at the Department of Medical Biochemistry and Biophysics, one of the researchers behind the study. "Information on the structure of the transport protein facilitates the development of better drugs in a shorter time. Such GLUT inhibitors could potentially be used to treat cancer in the future."
The study may be of significance not just to cancer research but also in the field of diabetes. GLUT plays a key role in diabetes since insulin works by activating the uptake of glucose from the blood by means of GLUT transporters in the cell membrane.
GLUT and the studied XylE transporter belong to the very large group of metabolite transporters called the Major Facilitator Superfamily (MFS), which is important in many diseases and for the uptake of medicines in cells.
"Many aspects concerning molecular mechanisms for the function of GLUT transporters are probably common to many members of the MFS family, which are involved in a broad spectrum of diseases in addition to cancer and diabetes," says Pär Nordlund.
As well as membrane transporters, which have undergone in-depth analysis in the current study, many different membrane proteins pass through the surface membrane of the cells. Their significance to the cell function and the development of drugs has been noted before, not least through the Nobel Prizes that were awarded to researchers who used mechanistic and structural studies to map the function of two other major membrane protein families, G-protein-coupled receptors and ion channels.
More information: 'Structural basis for substrate transport in the GLUT homology family of monosaccharide transporters', Esben M. Quistgaard, Christian Löw, Per Moberg, Lionel Trésaugues, and Pär Nordlund, Nature Structural & Molecular Biology, online 28 April 2013, doi: 10.1038/nsmb.2569